Bronchopulmonary dysplasia (BPD) is a chronic lung disease that mainly affects extremely pre-term infants, and remains the most common complication of prematurity. Several studies have shown that prematurity predisposes to the development of asthma in school children and adolescents. Nevertheless, it is not clear to what extent a history of BPD involves an additional risk.
MethodsA systematic review of studies assessing the association between BPD and asthma in school-children and adolescents was made. A literature search was carried out in the MEDLINE and EMBASE databases to retrieve articles published between 1 January 2000 and 31 August 2016.
ResultsA total of 17 studies comprising 7433 patients were included in the review. There was considerable heterogeneity in the definitions of BPD and asthma among studies. Overall, the prevalence of asthma was higher in children and adolescents with a history of prematurity and BPD compared with those who did not develop BPD. However, in only one of the studies did this difference reach statistical significance. The main limitation of this review was potential bias due to the lack of adjustment for confounding factors between exposure (BPD) and outcome (asthma) in most of the studies.
ConclusionBased on the studies reviewed, it cannot be argued that BPD, as an independent factor of prematurity, increases the risk of asthma defined by clinical parameters in school-children and adolescents. Further studies of greater methodological quality and homogeneous diagnostic criteria of BPD and asthma are needed for improved assessment of this association.
Bronchopulmonary dysplasia (BPD) is a chronic lung disease characteristic of pre-term infants (PTIs) with very low weight at birth (VLWB), particularly infants weighing less than 1000g, or with extremely low weight at birth (ELWB), and constitutes the most frequent complication of prematurity.1,2 Despite continuous advances in the care of PTIs, the prevalence of BPD still reaches 40% among infants with a gestational age (GA) of under 29 weeks.3
As initially described, BPD affected PTIs with a GA of over 32 weeks exposed to aggressive mechanical ventilation and high concentrations of oxygen (i.e., “classical” BPD).4 With new ventilation techniques, the administration of surfactant, and corticosteroid use in pregnant women, BPD currently affects particularly extreme PTIs (GA≤28 weeks) with lung development between the canalicular and saccular phases. In contrast to the important inflammatory and fibrotic component inherent to the classical forms of BPD, the disease in its current typical presentation is basically regarded as a consequence of the interruption of vascular and lung development1 (i.e., “new” BPD).
Bronchopulmonary dysplasia can predispose to the development of obstructive pulmonary diseases as a consequence of its effect upon lung and airway growth. In nursing and pre-school infants, BPD is a cause of great respiratory morbidity, with frequent admissions to hospital and respiratory exacerbations in most cases associated to viral infections.5 From the functional perspective, the condition typically involves important reductions in expiratory flow with air trapping.6,7 School-children and adolescents with a history of BPD continue to experience alterations in lung function. Such patients show a persistent obstructive pattern with air trapping, bronchial hyperresponsiveness following stimulation with methacholine and after physical exercise, and a diminished carbon monoxide diffusion capacity.7 In parallel to these findings, these subjects can be expected to exhibit a greater prevalence of asthmatic symptoms at these ages.
To date, three systematic reviews with meta-analyses have been published on the possible association between prematurity and asthma.8–10 Although the studies included in the reviews are heterogeneous in their definition of asthma, the age of the patients (from nursing infants to adolescents), and the contemplated birth period (before or after the introduction of pre-natal surfactant and corticosteroids), all conclude that premature delivery increases the risk of asthma over the short and long term. According to the meta-analyses, the risk of asthma is inversely proportional to GA. This could suggest that patients with BPD – the immense majority of which correspond to extreme prematurity cases – are at a greater risk of suffering asthma than the rest of PTIs. However, none of the reviews have specifically analysed this issue, and at present it is not possible to affirm whether BPD is an independent risk factor for asthma in school-children and adolescents.
A systematic review has recently been published on studies of the evolution of patients with BPD from the nursing infant stage to adulthood.11 However, most of the studies in school children and adolescents focus on lung function, with very little analysis of the prevalence of asthma from the clinical perspective.
Beyond the consequences of BPD in the first years of life, when the effects of prematurity and lung injuries are more evident, it is also important to know its clinical repercussions in later stages. At these ages the capacity to repair the damage caused may be evidenced in part while lung development and growth progresses.
The present study offers a systematic review of the publications that have investigated the possible association between BPD, as it is understood today, and asthma in school-children and adolescents, attempting to view BPD as an independent risk factor for asthma associated to prematurity.
MethodsSearch strategyA literature search was made of the MEDLINE and EMBASE databases, using combinations of the following as key words: “bronchopulmonary dysplasia”, “chronic lung disease”, “asthma”, “prematurity”, “very low birth weight” and “outcomes”. We selected only those studies that included clinical information on school-children and/or adolescents and were published from the year 2000 onwards, in order to increase the likelihood that the study subjects had been born in a period when pre-natal corticosteroids and surfactants were already in use (i.e., “new” BPD).
Inclusion criteria and screening of studiesOnly studies published in English and with access to the full text article were included. Journal editorials, congress summaries, and systematic reviews or meta-analyses were not taken into account.
We excluded all those studies that failed to specify whether the patients presented BPD (or prematurity chronic lung disease), or which did not clearly state the criteria defining the disorder. We likewise excluded those studies that failed to specify the association between BPD and asthma in some way, i.e., as the prevalence of asthma in the studied populations, or based on some risk index of one population with respect to another. Studies without at least one control group – whether full-term infants (FTIs) or PTIs – were excluded.
The existence of any of the following in the year before the study was accepted as representing a diagnosis of asthma: a medical diagnosis of asthma, asthma symptoms questionnaires addressed to the parents and/or patients, the use of anti-asthma medication and/or positive bronchodilator or bronchial provocation testing. When one same study used several of the above, priority was given to a medical diagnosis of asthma. A history of wheezing episodes at some point in time (“ever” asthma) was not taken to represent asthma if the patient had been free of such episodes in the last year.
Following the initial search, we selected the potentially eligible studies by reading the abstracts. We subsequently reviewed the full-text version of each publication and checked compliance with the inclusion criteria for definitive selection. Lastly, we examined the literature reference lists of the selected articles in search of additional studies.
The study selection process was carried out by three investigators. In a first phase a balanced distribution was made of the articles obtained in the search for evaluation on a separate basis. The inclusion criteria of each study were then jointly discussed to allow definitive selection.
Data extractionThe following information was extracted from each study: year of publication, study design, patient birth period and age at the time of the study, sample size of each of the studied populations, measure of association, measures of lung function, BPD criteria, and the criteria used to define asthma. In the case of those studies that did not provide the odds ratio (OR) of asthma between patients with and without BPD, calculation was made of the raw OR with the corresponding 95% confidence interval (95%CI). To this effect, 2×2 contingency tables were made based on the prevalence of asthma of each population (expressed as a percentage) and its sample size.
Study qualityThe study quality was assessed using the “Quality assessment tool for observational cohort and cross-sectional studies” of the National Heart, Lung and Blood Institute (NIH).12
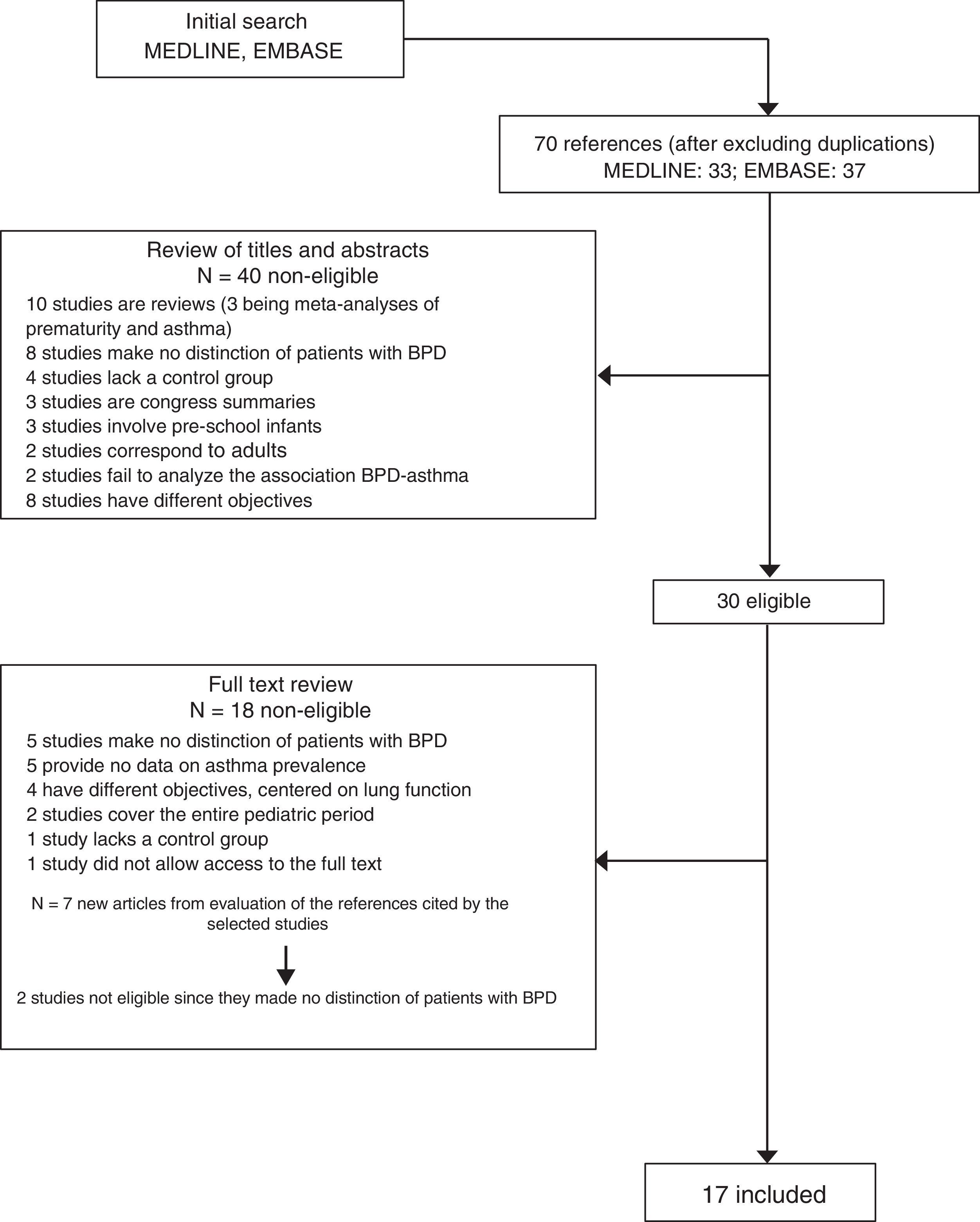
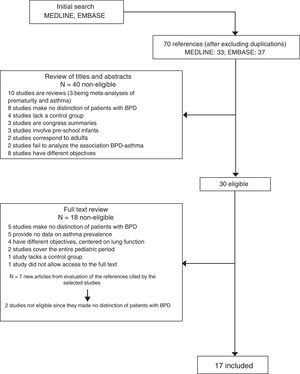
ResultsThe MEDLINE and EMBASE search identified 70 articles (Fig. 1). After reviewing the abstracts, 40 studies were considered irrelevant to the effects of our review or failed to meet the inclusion criteria, and were thus rejected. Following evaluation of the full text articles, an additional 18 publications were likewise excluded because they failed to meet the established criteria. Examination of the references cited in the selected articles yielded an additional seven studies of which two were rejected. The final review was thus based on 17 studies13–29, which are summarised in Table 1. Overall, the selected studies included a total of 1402 patients with a diagnosis of BPD in a global population of 7433 PTIs with a gestational age of under 32 weeks and/or with a weight at birth of less than 1500g, born in the period between 1977 and 2005. Seven studies included a control group of FTIs.14,16,17,20,22,24,25
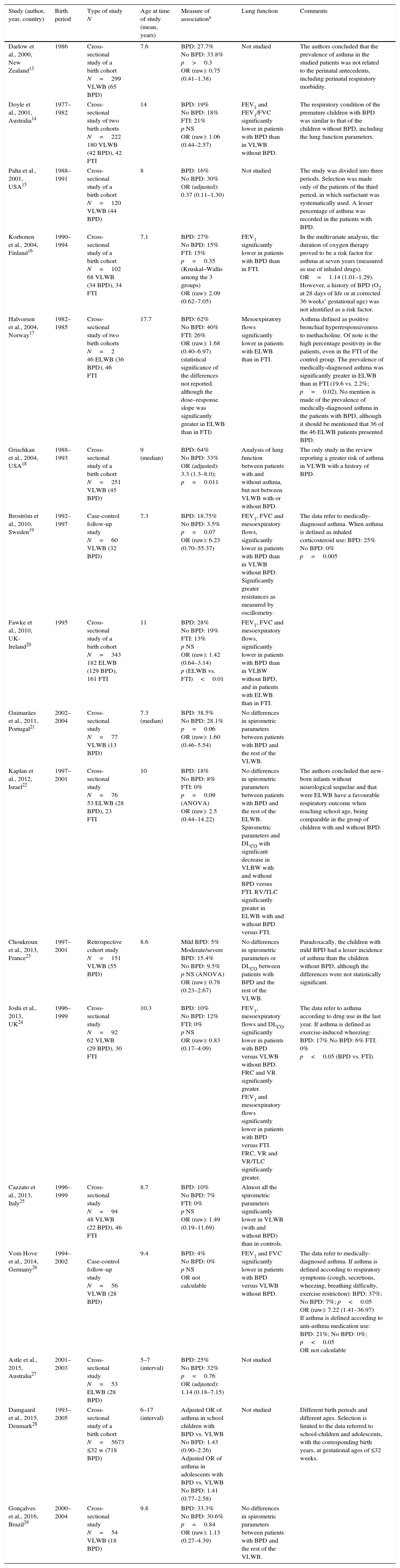
Association between BPD and asthma in school-children and adolescents. Summary of the studies included in the review.
| Study (author, year, country) | Birth period | Type of study N | Age at time of study (mean, years) | Measure of associationa | Lung function | Comments |
|---|---|---|---|---|---|---|
| Darlow et al., 2000, New Zealand13 | 1986 | Cross-sectional study of a birth cohort N=299 VLWB (65 BPD) | 7.6 | BPD: 27.7% No BPD: 33.8% p>0.3 OR (raw): 0.75 (0.41–1.38) | Not studied | The authors concluded that the prevalence of asthma in the studied patients was not related to the perinatal antecedents, including perinatal respiratory morbidity. |
| Doyle et al., 2001, Australia14 | 1977–1982 | Cross-sectional study of two birth cohorts N=222 180 VLWB (42 BPD), 42 FTI | 14 | BPD: 19% No BPD: 18% FTI: 21% p NS OR (raw): 1.06 (0.44–2.57) | FEV1 and FEV1/FVC significantly lower in patients with BPD than in VLWB without BPD. | The respiratory condition of the premature children with BPD was similar to that of the children without BPD, including the lung function parameters. |
| Palta et al., 2001, USA15 | 1988–1991 | Cross-sectional study of a birth cohort N=120 VLWB (44 BPD) | 8 | BPD: 16% No BPD: 30% OR (adjusted): 0.37 (0.11–1.30) | Not studied | The study was divided into three periods. Selection was made only of the patients of the third period, in which surfactant was systematically used. A lesser percentage of asthma was recorded in the patients with BPD. |
| Korhonen et al., 2004, Finland16 | 1990–1994 | Cross-sectional study of a birth cohort N=102 68 VLWB (34 BPD), 34 FTI | 7.1 | BPD: 27% No BPD: 15% FTI: 15% p=0.35 (Kruskal–Wallis among the 3 groups) OR (raw): 2.09 (0.62–7.05) | FEV1 significantly lower in patients with BPD than in FTI. | In the multivariate analysis, the duration of oxygen therapy proved to be a risk factor for asthma at seven years (measured as use of inhaled drugs). OR=1.14 (1.01–1.29). However, a history of BPD (O2 at 28 days of life or at corrected 36 weeks’ gestational age) was not identified as a risk factor. |
| Halvorsen et al., 2004, Norway17 | 1982–1985 | Cross-sectional study of two birth cohorts N=2 46 ELWB (36 BPD), 46 FTI | 17.7 | BPD: 62% No BPD: 40% FTI: 26% OR (raw): 1.68 (0.40–6.97) (statistical significance of the differences not reported. although the dose–response slope was significantly greater in ELWB than in FTI) | Mesoexpiratory flows significantly lower in patients with ELWB than in FTI. | Asthma defined as positive bronchial hyperresponsiveness to methacholine. Of note is the high percentage positivity in the patients, even in the FTI of the control group. The prevalence of medically-diagnosed asthma was significantly greater in ELWB than in FTI (19.6 vs. 2.2%; p=0.02). No mention is made of the prevalence of medically-diagnosed asthma in the patients with BPD, although it should be mentioned that 36 of the 46 ELWB patients presented BPD. |
| Grischkan et al., 2004, USA18 | 1988–1993 | Cross-sectional study of a birth cohort N=251 VLWB (45 BPD) | 9 (median) | BPD: 64% No BPD: 33% OR (adjusted): 3.3 (1.3–8.0); p=0.011 | Analysis of lung function between patients with and without asthma, but not between VLWB with or without BPD. | The only study in the review reporting a greater risk of asthma in VLWB with a history of BPD. |
| Broström et al., 2010, Sweden19 | 1992–1997 | Case-control follow-up study N=60 VLWB (32 BPD) | 7.3 | BPD: 18.75% No BPD: 3.5% p=0.07 OR (raw): 6.23 (0.70–55.37) | FEV1, FVC and mesoexpiratory flows, significantly lower in patients with BPD than in VLWB without BPD. Significantly greater resistances as measured by oscillometry. | The data refer to medically-diagnosed asthma. When asthma is defined as inhaled corticosteroid use: BPD: 25% No BPD: 0% p=0.005 |
| Fawke et al., 2010, UK-Ireland20 | 1995 | Cross-sectional study of a birth cohort N=343 182 ELWB (129 BPD), 161 FTI | 11 | BPD: 28% No BPD: 19% FTI: 13% p NS OR (raw): 1.42 (0.64–3.14) p (ELWB vs. FTI)<0.01 | FEV1, FVC and mesoexpiratory flows, significantly lower in patients with BPD than in VLBW without BPD, and in patients with ELWB than in FTI. | |
| Guimaräes et al., 2011, Portugal21 | 2002–2004 | Cross-sectional study N=77 VLWB (13 BPD) | 7.3 (median) | BPD: 38.5% No BPD: 28.1% p=0.06 OR (raw): 1.60 (0.46–5.54) | No differences in spirometric parameters between patients with BPD and the rest of the VLWB. | |
| Kaplan et al., 2012, Israel22 | 1997–2001 | Cross-sectional study N=76 53 ELWB (28 BPD), 23 FTI | 10 | BPD: 18% No BPD: 8% FTI: 0% p=0.09 (ANOVA) OR (raw): 2.5 (0.44–14.22) | No differences in spirometric parameters between patients with BPD and the rest of the ELWB. Spirometric parameters and DLCO with significant decrease in VLBW with and without BPD versus FTI. RV/TLC significantly greater in ELWB with and without BPD versus FTI. | The authors concluded that new-born infants without neurological sequelae and that were ELWB have a favourable respiratory outcome when reaching school age, being comparable in the group of children with and without BPD. |
| Choukroun et al., 2013, France23 | 1997–2001 | Retrospective cohort study N=151 VLWB (55 BPD) | 8.6 | Mild BPD: 5% Moderate/severe BPD: 15.4% No BPD: 9.5% p NS (ANOVA) OR (raw): 0.78 (0.23–2.67) | No differences in spirometric parameters or DLCO between patients with BPD and the rest of the VLWB. | Paradoxically, the children with mild BPD had a lesser incidence of asthma than the children without BPD, although the differences were not statistically significant. |
| Joshi et al., 2013, UK24 | 1996–1999 | Cross-sectional study N=92 62 VLWB (29 BPD), 30 FTI | 10.3 | BPD: 10% No BPD: 12% FTI: 0% p NS OR (raw): 0.83 (0.17–4.09) | FEV1, mesoexpiratory flows and DLCO significantly lower in patients with BPD versus VLWB without BPD. FRC and VR significantly greater. FEV1 and mesoexpiratory flows significantly lower in patients with BPD versus FTI. FRC, VR and VR/TLC significantly greater. | The data refer to asthma according to drug use in the last year. If asthma is defined as exercise-induced wheezing: BPD: 17% No BPD: 6% FTI: 0% p<0.05 (BPD vs. FTI) |
| Cazzato et al., 2013, Italy25 | 1996–1999 | Cross-sectional study N=94 48 VLWB (22 BPD), 46 FTI | 8.7 | BPD: 10% No BPD: 7% FTI: 0% p NS OR (raw): 1.49 (0.19–11.69) | Almost all the spirometric parameters significantly lower in VLWB (with and without BPD) than in controls. | |
| Vom Hove et al., 2014, Germany26 | 1994–2002 | Case-control follow-up study N=56 VLWB (28 BPD) | 9.4 | BPD: 4% No BPD: 0% p NS OR not calculable | FEV1 and FVC significantly lower in patients with BPD versus VLWB without BPD. | The data refer to medically-diagnosed asthma. If asthma is defined according to respiratory symptoms (cough, secretions, wheezing, breathing difficulty, exercise restriction): BPD: 37%; No BPD: 7%; p<0.05 OR (raw): 7.22 (1.41–36.97) If asthma is defined according to anti-asthma medication use: BPD: 21%; No BPD: 0%; p<0.05 OR not calculable |
| Astle et al., 2015, Australia27 | 2001–2003 | Cross-sectional study N=53 ELWB (28 BPD) | 5–7 (interval) | BPD: 25% No BPD: 32% p=0.76 OR (adjusted): 1.14 (0.18–7.15) | Not studied | |
| Damgaard et al., 2015, Denmark28 | 1993–2005 | Cross-sectional study of a birth cohort N=5673 ≤32 w (718 BPD) | 6–17 (interval) | Adjusted OR of asthma in school children with BPD vs. VLWB No BPD: 1.43 (0.90–2.26) Adjusted OR of asthma in adolescents with BPD vs. VLWB No BPD: 1.41 (0.77–2.58) | Not studied | Different birth periods and different ages. Selection is limited to the data referred to school-children and adolescents, with the corresponding birth years, at gestational ages of ≤32 weeks. |
| Gonçalves et al., 2016, Brazil29 | 2000–2004 | Cross-sectional study N=54 VLWB (18 BPD) | 9.8 | BPD: 33.3% No BPD: 30.6% p=0.84 OR (raw): 1.13 (0.27–4.39) | No differences in spirometric parameters between patients with BPD and the rest of the VLWB. | |
The percentages express prevalence of asthma in the studied populations. The odds ratios (OR) express risk of asthma in prematurity with BPD versus prematurity without BPD. Statistical significance, except where stated otherwise, refers to differences between premature patient with and without BPD.
FEV1: forced expiratory volume in 1s; FVC: forced vital capacity; RV: residual volume; TLC: total lung capacity; FRC: functional residual capacity; DLco: carbon monoxide diffusing capacity; NS: non-significant; ELWB: extremely low weight at birth; VLWB: very low weight at birth.
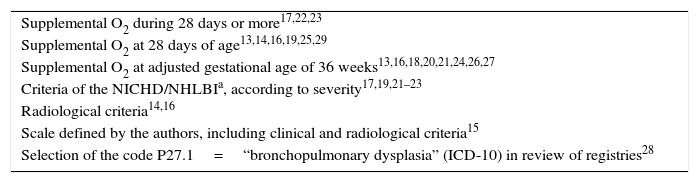
The most frequently used criterion for defining BPD was the need for oxygen (O2) with a corrected GA of 36 weeks, although great heterogeneity was observed among the studies (Table 2). In most cases, the diagnosis of asthma was based on the results of questionnaires referred to symptoms and/or the use of drugs in the year before the study (Table 3).
Diagnostic criteria of BPD used in the selected studies.
| Supplemental O2 during 28 days or more17,22,23 |
| Supplemental O2 at 28 days of age13,14,16,19,25,29 |
| Supplemental O2 at adjusted gestational age of 36 weeks13,16,18,20,21,24,26,27 |
| Criteria of the NICHD/NHLBIa, according to severity17,19,21–23 |
| Radiological criteria14,16 |
| Scale defined by the authors, including clinical and radiological criteria15 |
| Selection of the code P27.1=“bronchopulmonary dysplasia” (ICD-10) in review of registries28 |
National Institute of Child Health and Human Development/National Heart, Lung and Blood Institute.30
Diagnostic criteria of asthma used in the selected studies.
| Medical diagnosis of asthma18–20,24–26 |
| Results of asthma questionnaires based on ISAAC methodologya.15,24,29 |
| Results of other asthma questionnaires13,14,18,21–23,26,27 |
| Diagnosis based on the use of drugs for asthma13,14,16,18,26,28 |
| Bronchial hyper-responsiveness to methacholine17 |
In general terms, the prevalence of asthma in the last year was greater among the patients with a history of BPD than in the rest of the PTIs, although in only one study was the difference seen to be statistically significant.18 On applying broader criteria of asthma (i.e., use of drugs or appearance of symptoms with physical exercise) instead of stricter criteria (medically-diagnosed asthma), an additional three studies recorded significant differences in favour of the patients with BPD.19,24,26 In contrast, four studies found the prevalence of asthma to be slightly greater among the PTIs without BPD.13,15,24,27
Of the five studies that used a classification of BPD based on severity,17,19,21–23 only two compared the prevalence of asthma taking the disease stage into account.17,23 The differences between moderate or severe stage BPD versus mild BPD or even pre-term status without BPD were not statistically significant.
The studies that included FTIs as controls likewise found no significant differences in asthma prevalence on establishing comparisons versus the patients diagnosed with BPD. In contrast, two publications found the prevalence of asthma to be significantly greater in the overall PTIs with ELWB than among the FTIs.17,20
Although our search was limited to studies published from the year 2000 onwards, some of them included patients born before the systematic use of pre-natal corticosteroids or the introduction of surfactant in Neonatal Intensive Care Units (NICUs).13–15,17,18 However, among the patients diagnosed with BPD and born in that early period, the prevalence of asthma was no greater than in those born after the introduction of surfactant.
Lung functionMost of the studies supplied information on lung function (Table 1). Although some of them reported greater alterations in the spirometric parameters among the patients with BPD versus the rest of the PTIs,14,19,20,24,26 others failed to observe significant differences.21–23,29 All the studied FTI controls exhibited significantly better lung function than the PTIs, regardless of whether they had BPD16,22,24 or not.17,20,22,25
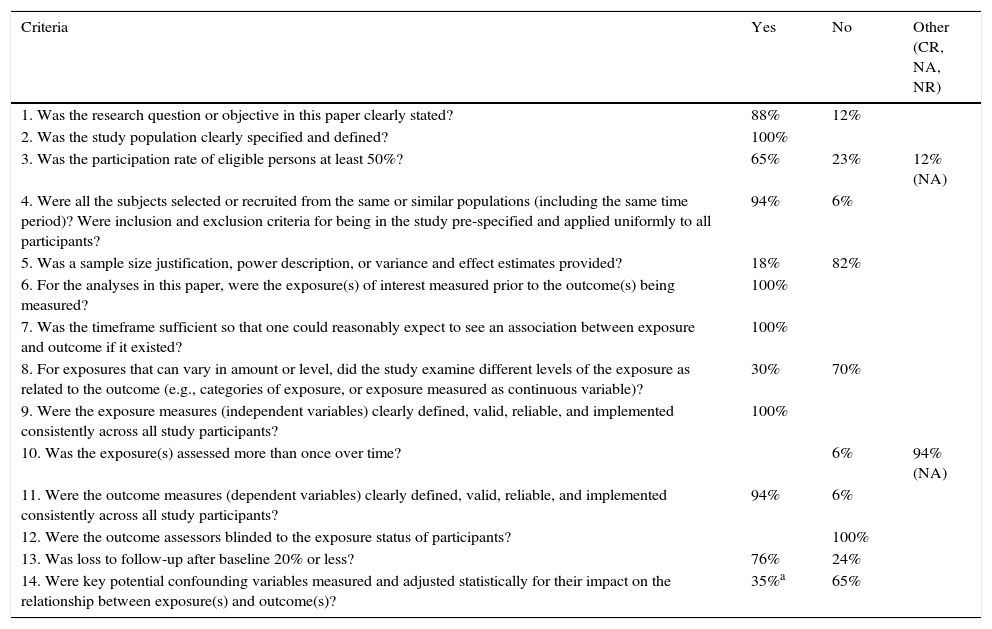
Study quality. Potential sources of biasIn the immense majority of the studies included in our review, the sources of bias were found to be (Table 4): lack of justification of the sample size and of the power of the study; failure to include the different levels of exposure according to categories (BPD severity grades); lack of investigator masking with regard to the variable exposure; and a lack of measurement and statistical adjustment of the possible confounding factors between the variable exposure (BPD) and the variable outcome (asthma). Only four studies reported the adjusted OR between BPD and asthma. All of them took GA, weight at birth, smoking and a history of asthma and/or atopy in the family into account.15,18,27,28
Quality assessment tool for observational cohort and cross-sectional studies.13
| Criteria | Yes | No | Other (CR, NA, NR) |
|---|---|---|---|
| 1. Was the research question or objective in this paper clearly stated? | 88% | 12% | |
| 2. Was the study population clearly specified and defined? | 100% | ||
| 3. Was the participation rate of eligible persons at least 50%? | 65% | 23% | 12% (NA) |
| 4. Were all the subjects selected or recruited from the same or similar populations (including the same time period)? Were inclusion and exclusion criteria for being in the study pre-specified and applied uniformly to all participants? | 94% | 6% | |
| 5. Was a sample size justification, power description, or variance and effect estimates provided? | 18% | 82% | |
| 6. For the analyses in this paper, were the exposure(s) of interest measured prior to the outcome(s) being measured? | 100% | ||
| 7. Was the timeframe sufficient so that one could reasonably expect to see an association between exposure and outcome if it existed? | 100% | ||
| 8. For exposures that can vary in amount or level, did the study examine different levels of the exposure as related to the outcome (e.g., categories of exposure, or exposure measured as continuous variable)? | 30% | 70% | |
| 9. Were the exposure measures (independent variables) clearly defined, valid, reliable, and implemented consistently across all study participants? | 100% | ||
| 10. Was the exposure(s) assessed more than once over time? | 6% | 94% (NA) | |
| 11. Were the outcome measures (dependent variables) clearly defined, valid, reliable, and implemented consistently across all study participants? | 94% | 6% | |
| 12. Were the outcome assessors blinded to the exposure status of participants? | 100% | ||
| 13. Was loss to follow-up after baseline 20% or less? | 76% | 24% | |
| 14. Were key potential confounding variables measured and adjusted statistically for their impact on the relationship between exposure(s) and outcome(s)? | 35%a | 65% |
The boxes express the percentage of studies for each of the possible responses. CR: cannot determine; NA: not applicable; NR: not reported.
To the best of our knowledge, this is the first review to specifically analyse the association between BPD and asthma in school-children and adolescents. The evaluated studies suggest a discretely higher prevalence of asthma in patients with BPD versus the rest of PTIs without BPD. However, in only one study was the difference seen to be statistically significant.18
The first aspect to be commented in our review is the great heterogeneity among studies in terms of the criteria used to define BPD and asthma.
The variability in the definition of BPD is partly conditioned by the changes that have taken place in the diagnostic criteria during the period covered by our review. The most widely used criterion for BPD was the need for supplementary oxygen at a corrected GA of 36 weeks. The inconvenience of this definition is that a proportion of children requiring prolonged supplemental oxygen are not diagnosed with BPD if the oxygen requirements end before the age of 36 weeks – although this does not free them from possible respiratory sequelae. On examining the prevalence of asthma in these patients over the following years, there might be no significant differences versus the patients diagnosed with BPD. Therefore, the National Institute of Child Health and Human Development (NICHD) consensus defined BPD as the need for supplemental oxygen during at least 28 days – classifying the condition as mild, moderate or severe according to the oxygen or ventilatory support requirements at a corrected GA of 36 weeks.30 It would be expected that children with a diagnosis of severe BPD have a greater prevalence of asthma, yet this cannot be confirmed from the reviewed articles, since only two of them took severity into account on establishing comparisons.7,23
However, some authors question whether the commonly-used definitions of BPD are a good indicator of long-term respiratory outcome.11,32 In fact, some therapeutic strategies used in the neonatal phase, while exerting no impact upon the incidence of BPD over the short term, do reduce respiratory morbidity later on in life.33,34 In contrast, other preventive measures that have been able to reduce the incidence of BPD do not seem to result in changes in respiratory outcome when comparing patients with and without a diagnosis of BPD.35 Lastly, factors such as atopy in the family, viral infections, and exposure to tobacco smoke and other environmental agents may largely condition the outcome in patients with a history of prematurity and VLWB, independently of the existence of a prior diagnosis of BPD.
Heterogeneity among the studies has also been observed regarding the definition of asthma. Some authors have even used more than one definition to classify their patients. Depending on the definition used, one same study can report considerable differences in the prevalence of asthma.19,24,26
There is controversy as to whether the respiratory symptoms in school-children or adolescents with a history of BPD can be defined as asthma, or whether BPD and asthma should be regarded as different entities.36–39 In effect, both disease conditions share a number of characteristics such as an inflammatory basis, bronchial hyperresponsiveness, and chronic airflow obstruction. However, there are also differences that must be taken into account. While the predominant cells in asthmatic inflammation are eosinophils and mast cells, with a cytokine profile inherent to Th2-mediated responses, BPD is characterised by a predominance of macrophages and neutrophils, and the cytokine profile in this case corresponds to a Th1-mediated response.39 In this respect, it has been shown that children with bronchial obstruction associated to BPD have significantly lower levels of exhaled NO – an eosinophilic inflammation marker typical of asthma – than patients with asthma.40,36 On the other hand, both diseases are characterised by bronchoconstriction following provocation with methacholine as a non-specific marker of bronchial hyperresponsiveness. However, only asthmatic patients with an atopic basis exhibit a positive response after administering adenosine 5′-monophosphate.41,42 In contrast to what is seen in asthmatics, most patients with BPD do not respond to bronchodilation testing – this having been attributed to an early bronchial remodelling process.36 Lastly, the condition of atopy, characteristic of asthma in school-children and adolescents, is not seen more frequently in patients with a history of BPD than in the rest of the population.26,27,43,44 Some studies have even shown that children that were born prematurely have a lesser incidence of atopic disorders over the long term.16,45 In the present review we aimed to compile information on the prevalence of asthma symptoms – generally diagnosed by a physician or from questionnaires – as indicators of respiratory morbidity, without considering the differential aspects between BPD and asthma commented above.
Little information is available on the clinical outcome in school-children and adolescents with BPD born before the introduction of pre-natal corticosteroids and surfactant, since the studies focused particularly on the evaluation of lung function.46–50 In our review, we observed no clear differences in the prevalence of asthma between the studies carried out before and after the introduction of these therapies. Only the study published by Palta et al., which included patients born in different periods, described significant improvement of the respiratory symptoms on comparing patients with BPD born before the introduction of surfactant versus patients born in the period when surfactant use had become routine practice.15
Of the studies that included a FTI control group, only two reported a significantly greater prevalence of asthma in school-children and adolescents with a history of prematurity, independently of the presence or not of BPD.17,20 Nevertheless, it must be mentioned that in the studies without an FTI control group, the prevalence of asthma in the overall premature population was almost always greater than in the general population (taking as reference the results of the ISAAC study for these ages in each country or geographical setting51). There is sufficient evidence to affirm that prematurity alone increases the risk of asthma in the course of the paediatric age.8–10 This association appears to be related to the lesser development of the lungs and airways, which in turn is a consequence of other factors associated to prematurity, such as inflammation, maternal smoking, hypoxia and intrauterine growth retardation. All these circumstances would increase airway responsiveness and result in chronic airflow obstruction. Lung function testing in school-children and adolescents with a history of prematurity evidences the persistence of these alterations, as has been observed in most of the studies included in our review. The question of whether the increase in asthma prevalence is due only to prematurity, or whether BPD constitutes an additional risk factor, cannot be answered on the basis of the reviewed studies.
In addition to the heterogeneity of the definitions of asthma and BPD, the present review has other limitations related to the methodology of the studies. Most of the articles involved a cross-sectional design in which the primary objective was evaluation of the clinical condition over the long term, and particularly respiratory function in the global population of premature patients with VLWB or ELWB. From the clinical perspective, the data provided almost always consisted of simple percentages reflecting the prevalence of asthma in sub-groups of patients with and without BPD, coded as raw measures of asthma risk in the form of OR. Few studies adequately measured and/or adjusted the differences between patients with and without BPD in relation to the main confounding factors. Some of them, such as GA, exposure to tobacco smoke, or a history of atopy in the family or in the patient, can exert a considerable influence upon the prevalence of asthma. Of the factors shown in Table 4, we consider this to be the main source of potential bias in interpreting the results.
ConclusionsBased on the reviewed studies, it cannot be affirmed that BPD, as an independent prematurity factor, increases the risk of asthma defined on the basis of clinical parameters in school-children and adolescents. In order to more precisely establish the possible association, it would be advisable to conduct prospective studies using homogenous diagnostic criteria referred to BPD (based on degree of severity) and asthma, taking into account the need for adequate measurement and adjustment of the possible confounding factors.
On the other hand, the alterations in lung function of these patients must be taken into consideration. It is therefore advisable to carry out long-term follow-up of these individuals due to the risk of developing chronic obstructive pulmonary disease in adult life.
Ethical disclosuresConfidentiality of dataThe authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
Protection of human subjects and animals in researchThe authors declare that no experiments were performed on humans or animals for this investigation.
Conflict of interestThe authors have no conflict of interest to declare.