INTRODUCTION
Allergic reactions to cow's milk proteins represents a significant problem in children in the first year of life. Currently, only studies including challenge tests (1-3) can be considered valid because diagnosis of allergy to cow's milk proteins implies a very restrictive diet for infants and children (3, 4).
We performed a prospective study of children diagnosed with allergy to cow's milk proteins. The aim of this study was to evaluate the different clinical reactions to challenge testing with cow's milk proteins at diagnosis and again after these infants had been fed an exclusion diet.
MATERIALS AND METHODS
Patients
Forty-nine consecutive infants aged less than 6 months old were included in the study. The infants had been referred to our allergy department with suspected allergy to cow's milk proteins and presented clinical signs and symptoms suggestive of immediate hypersensitivity reactions.
Methods
Immediate hypersensitivity to cow's milk proteins was diagnosed using the following criteria: a) a clinical history suggestive of allergy to cow's milk proteins with development of acute symptoms in the first 2 hours after ingestion of adapted formula; b) the presence of specific IgE antibodies evaluated through a positive skin prick test to one or more related proteins (alpha-lactalbumin, beta-lactoglobulin and/or casein) and the presence of serum specific IgE antibodies to one of more of these proteins; c) a positive controlled challenge test.
Skin prick testing was performed following a standarized procedure (5), with a -lactalbumin or b -lactoglobulin extracts (5 mg/ml) and casein (10 mg/ml) (all from CBF Leti, Barcelona, Spain). A result was considered positive when the test produced a skin weal of 3 mm or more in diameter.
Specific IgE antibodies to a -lactalbumin, b -lactoglobulin and casein were determined using UniCAP® (Pharmacia & Upjohn, Pharmacia Diagnostics, Uppsala, Sweden). Determination was considered positive when concentrations were > 0.35 KUI/l.
After the infants had fasted for 4 hours and after registration of vital signs (blood pressure and heart rate, performed with a Dynamap(TM) XL, breathing rate and clinical status) open challenge was carried out (5, 6) by administering up to 20 ml of adapted cow's milk protein formula (Nidina 1®, Nestle, Spain). Vital signs were monitored every 30 minutes during the first hour after formula intake and again every hour for 3 hours after the last dose administered in the challenge test. The infants were monitored for 24 hours after the challenge test.
The same methodology was repeated when the infants were aged 1 year old but challenge test was performed only in infants in whom immunological study was negative.
RESULTS
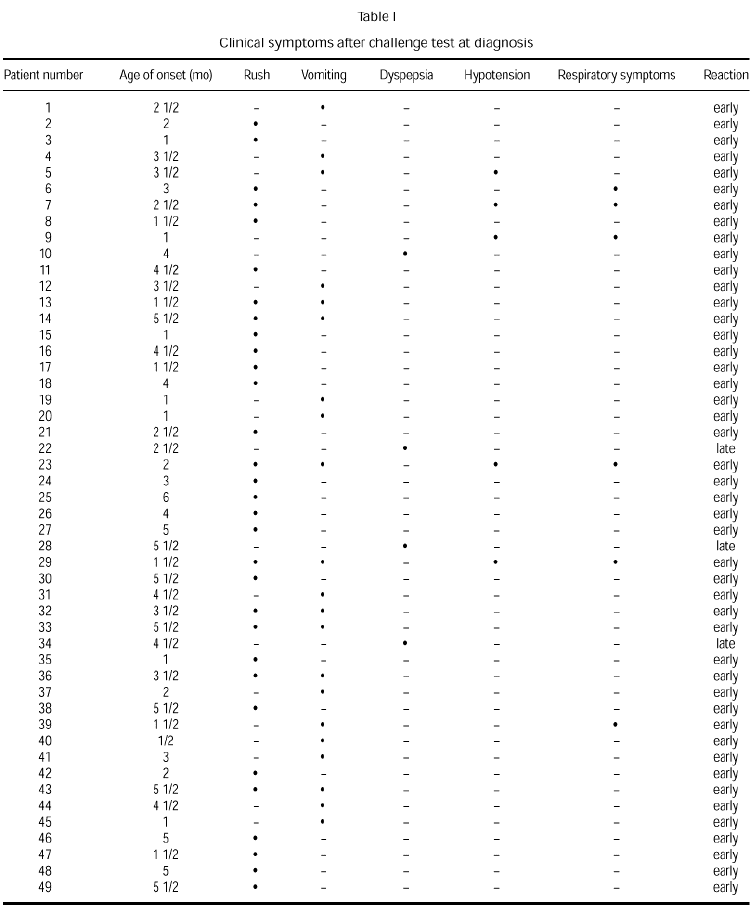
At diagnosis, challenge testing provoked immediate reactions in 92 % of the children studied. Symptoms found after the test were rush in 63 %, vomiting in 43 %, anaphylaxis in 10 % and respiratory symptoms in 12 %. Only 8 % of the clinical reactions observed after challenge testing were late and all manifested as dyspepsia. Respiratory symptoms were never isolated and always appeared concomitantly. Some patients showed more than one symptom (table I).
All reactions after a positive challenge test were well controlled and in most cases the challenge was stopped. Some infants required treatment with oral antihistamine; all anaphylactic reactions required adrenaline treatment. Systemic corticoids were required in only 3 patients.
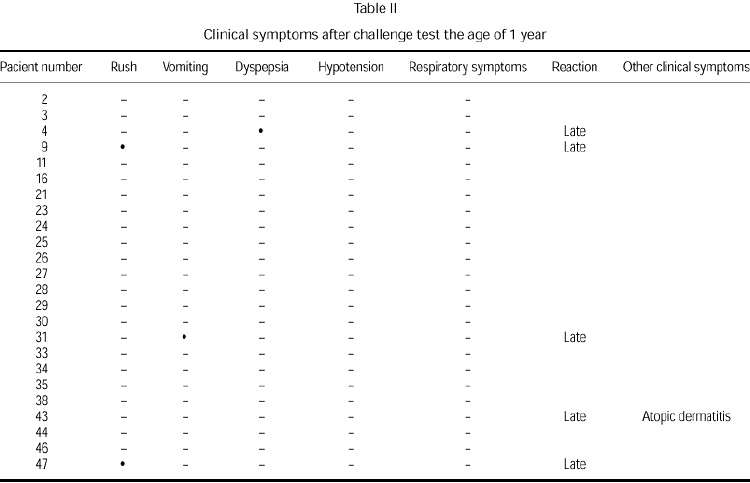
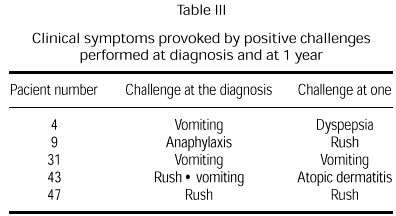
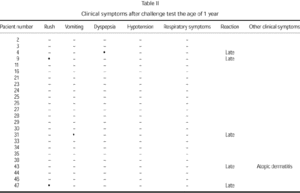
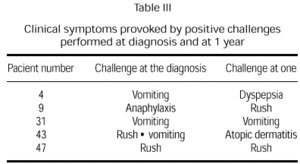
Clinical manifestations after challenge testing in patients at the age of 1 year with negative immunological reactions to cow's milk proteins [(skin prick test, radioallergosorbent test (RAST)] are presented in table II. Only five children (21 %) presented positive reaction to challenge testing. Clinical manifestations never presented in the first 24 hours after the reintroduction of cow's milk protein to the diet. Two children showed clinical manifestations similar to those produced by the challenge test performed at diagnosis (table III).
DISCUSSION
We chose the open challenge test to study children with suspected allergy to Cow's milk protein (3, 6-8) because this test is accepted as a valid diagnostic procedure in such patients. Repetition of the challenge test after an exclusion diet is often the only means of confirming tolerance (4) because skin testing (and serum IgE) may remain positive for years after tolerance has been achieved.
Some authors (8, 9) have described the need to perform challenge tests in areas with resuscitation equipment. In our study, 10 % of the patients required resuscitation, all of whom presented anaphylaxis.
After an exclusion diet, challenge testing (in the first 3 hours after milk ingestion) may fail to produce immediate hypersensitivity reactions (10) but manifestations may develop later. This reaction usually is IgE-dependent (rash, vomiting, etc.). In patients included in this study, 60 % of positive reactions were of this kind. Whether these reactions are indeed late or whether they are a different type of immediate reaction is a matter of debate (11). In the present study, this type of reaction appeared in 21 % of the children included.
CONCLUSIONS
Challenge testing for the diagnosis of hypersensitivity to cow's milk proteins produced immediate reactions in 92 % of patients. Ten percent of these reactions manifested as anaphylaxis and required both resuscitation equipment and personnel trained in treating allergic reactions in patients admitted to day clinics.
Although not completely risk-free, diagnostic challenge tests are safe when performed under the conditions described. Challenge testing provoked no anaphylactic reactions in children who had followed a diet free of cow's milk proteins for 6 months and who had a negative skin prick test and negative IgE.
In these circumstances the challenge test may be performed in an out-patient clinic although the patient must be followed-up for the first 15 days after the reintroduction of milk into the diet. In a significant number of patients (21 %), reactions may appear a few days after cessation of exclusion diet.