The association between genetic predisposition and environmental risk factors such as passive smoke in determining respiratory allergies is still uncertain; even less is known about the role played by passive smoking in influencing the success of therapy for rhinitis and allergic asthma.
ObjectiveThe purpose of this prospective, randomised study was to determine whether passive smoking influences the outcome of therapies in paediatric patients with allergic respiratory diseases.
MethodsThe study included 68 children (mean age 11.51 years; range: 5-17) suffering from perennial rhinitis and intermittent asthma monosensitised to Dermatophagoides. Thirty-four subjects were exposed to daily passive smoking in their families, 34 were not. The two groups have been then randomised to receive continuous treatment with cetirizine or SLIT for three years.
ResultsThere were 3/34 (8.8%) dropouts in the SLIT arm and 4/34 (11.7%) in the cetirizine arm. After three years, the patients exposed to passive smoking showed higher nasal eosinophilia, a worse clinical-symptomatic and pharmacological score with a worsened bronchial reactivity and functional indices of persistent asthma, regardless of how they had been treated. Nevertheless, SLIT prevented the worsening of all the clinical parameters more than the antihistamine alone either among the children exposed to smoking or not.
ConclusionsExposure to passive smoking in children suffering from respiratory allergies due to Dermatophagoides decreased the clinical response to both drug therapy and SLIT. Nonetheless, while the children submitted to drug therapy worsened or did not show any significant improvement, the ones treated with SLIT improved.
Respiratory allergic diseases are one of the major causes of morbidity in industrialised countries and their incidence is constantly rising.1 Bronchial asthma in particular, especially in children, has increased over the last 30 years to the extent that it is the major cause of hospitalisation in infancy.2 A variety of preventive strategies has been proposed to combat this trend, especially in very young children, and which focus on several risk factors, such as parental tobacco smoke; breastfeeding; the microclimate of the environment; and living with animals.3,4 With the exception of exposure to passive cigarette smoke, the scientific evidence on the actual role played by the other risk factors in causing asthma is still conflicting, so it is difficult to implement primary prevention programmes.5 Indeed, there is convincing experimental evidence that passive smoking affects asthma, wheezing episodes, bronchial hyperreactivity and also increases risk of sensitisation to the more common environmental pneumoallergens during infancy.6 Exposure to maternal passive smoking appears to be more significant, probably because it is the mother who has the closest contact with the child within the family.7 Despite that, the data on the role played by active smoking in the pathogenesis of atopic diseases are still conflicting. In fact, apparently there is no association between maternal smoking during pregnancy and increased neonatal risk of asthma, hay fever and atopic dermatitis, with the exception of wheezing.8 Indeed, actual exposure to cigarette smoke appears to be associated with a decreased risk in the onset of atopic diseases in smokers.9
The aim of this study was to determine whether the exposure to parental passive smoke (at least 20 cigarettes per day) could affect the efficacy of the therapies currently available for treating respiratory allergic diseases in children.
Material and methodsStudy designThe prospective, open label, randomised study included two groups, one exposed to parental passive smoke, and the other not exposed.
PatientsThe study evaluated a group of 68 children with perennial allergic rhinitis and mild intermittent asthma monosensitised to house dust mites (HDM), 34 of whom were exposed to passive smoking daily. The two groups, after an evaluation of the immuno-allergic and functional profile in washout conditions, were randomised in 2006 to receive continuous therapy with antiH1 (cetirizine 10mg/day) or SLIT for 3 years (2006-2008), ending up with two groups of exposed to passive smoking and not exposed, with 17 patients submitted to drugs and 17 to SLIT each.
All the patients presented with the following at baseline:
- 1)
Clinical profile of perennial rhinitis and mild intermittent asthma (FEV1>80% of the expected value) according to the ARIA and GINA guidelines;
- 2)
Positive MCh challenge for PD20FEV1 (or PD35Sgaw) <400μg;
- 3)
Nasal eosinophilia > 10%;
- 4)
RAST for DP and DF=or > II^ Class;
The study was approved by the ethical committee of the hospital and all patients’ parents signed an informed consent before their children entered the study.
TreatmentIn spring 2006, each of the two patient groups (n=34 per group), exposed to passive smoke or not, was randomised to receive (ratio 1:1) continuous therapy with cetirizine (n=17) 1mg/2.5 Kg/day (< 12 years) or 10mg/day (>12 years), or to SLIT against Dermatophagoides (n=17), with monomeric allergoid (Lais®, Lofarma, Milan, Italy). In addition, all the patients were allowed to take salbutamol by inhalation (100 mcg 1-2 puffs as needed) and nasal corticosteroids (budesonide 100μg, one puff per nostril once or twice per day as needed).
The SLIT was administered in accordance with the latest Position Paper on the subject,10 using the therapeutic protocol recommended by the manufacturer The oromucosal specific immunotherapy involved administering a mixture of monomeric allergenic extracts of Dermatophagoides spp. (Dermatophagoides pteronyssimus 50%, Dermatophagoides farinae 50%), in the following Allergy Unit (AU) doses: 25-100-300-1000 AU. The extract is standardised by means of RAST-inhibition in accordance with an internal standard. The treatment included a dose-ascending phase of 14 weeks during which each dose was taken three times per week in accordance with a schedule provided by the company, and a maintenance phase during which the maximum dose reached of 1,000 AU was taken once per week for the following three years. The cumulative annual average dose taken was approximately 60,000 AU.
The patients were re-evaluated after three years to determine whether there was a less clinical control of the cytomorphological and functional parameters by the two different treatment strategies (immunological and not) in the children exposed to passive smoking versus those who were not and to compare the effects of the treatment with SLIT versus those of the control arm (cetirizine).
DiagnosisThe prick tests were performed in accordance with international guidelines11 using standardised commercial extracts (Alk Abello, Lainate, Milan, Italy) for the following allergens: Dermatophagoides pteronyssimus; D. farinae; grass pollen; Artemisia; ragweed; Parietaria; dog and cat dander; birch; olive; Alternaria; and Cladosporium.
The respiratory function tests were performed with computerised spirometry together with plethysmography box to study specific conductance and resistance (Masterlab Yaeger, Wurtzburg, Germany). The MCh challenge was conducted using a dosimeter (Yaeger) activated by inhalatory effort with administration of increasing doses of MCh: 30-60-120-240-390-690-1,290μg.12,13 Before the test, the patients underwent a washout period of 48hours for beta-stimulant drugs. The eosinophil count in the nasal secretions was performed with a nasal swab (front nasal cavity). The material collected was smeared on to glass and dried, stained using the May Grundwald-Giemsa method, and read under an optical microscope with immersion lens. The eosinophil count (the number of eosinophils per 100 white globules counted in the nasal secretion) was classified either as mild (< 10%) or moderate-severe (> 10%). The eosinophil count was performed after a washout period of at least seven days for topical nasal corticosteroids.
Patient diariesAll the patients were instructed on how to keep a clinical diary properly to record the symptoms and consumption of drugs each month at the beginning and at the end (after three years) of the treatment during November and February (the period of peak exposure to household mites in our geographic area). The clinical efficacy of the treatment was evaluated by taking into consideration the following parameters: coughing; wheezing; dyspnoea; nasal obstruction; nasal pruritis; rhinorrhea; sneezing; conjunctival pruritis; conjunctival redness; and watery eyes. Each symptom was evaluated in accordance with the following scale: 0=absent, 1=mild, 2=moderate, 3=severe. The monthly values ranged between 0 and 900. An average monthly symptom score was also obtained during the observation period for statistical purposes. The consumption of symptomatic drugs was recorded separately (salbutamol: 1 puff=1 point, nasal budesonide: 1 puff (100μg per nostril=1 point). The patients also underwent outpatient clinical visits every six months and an allergologist was available by telephone on a daily basis on demand.
Statistical analysesSex ratio at baseline was tested with Pearson Chi-squared. The probability levels for Pearson Chi-Square were computed using a complete randomisation method (permutation or exact test; PExact) or by a Monte Carlo simulation based on 100,000 sampled tables (PMC) when computation by the permutation method was not possible.
Clinical parameter differences between the therapy treatment group (SPT and SLIT) and between exposure to passive smoke were tested using a Student's t test for homogeneous or for not homogeneous variances if required after Levene's test for variance homogeneity.
Two-way ANOVA for repeated measures have been used to test the effects of the treatments (TREAT: SLIT vs SPT) on each clinical parameter in the two groups of patients with differential exposure to passive smoke (SMEXP: Exposed vs not Exposed to passive smoke). A model for each parameter was build with TREAT and SMEXP as the main factors, TREAT*SMEXP as the interaction factor, and AGE as the covariate.
Logistic Regression analysis was used to estimate the effect of the considered factors (age, sex, treatment and passive smoke exposure) on the occurrence of new sensitisations. The final model has been selected using a backward stepwise selection procedure using the Likelihood ratio LR statistic to determine if a variable should be removed from the model. The goodness of fit of the final model has been tested using the Hosmer-Lemeshow goodness-of-fit statistic. The model performance has been estimated using the 2 Log Likelihood, the Cox and Snell R2 and the Nagelkerke's R2. The relevance of each parameter in the occurrence of new sensitisations has been estimated using the standardised regression coefficients and through the exp(B) that can be interpreted as an odds ratio for main effects model.
All the statistical analyses have been computed using the SYNTAX procedure, implemented in the Statistical Package for Social Sciences ver. 17.01 (SPSS® Inc.).
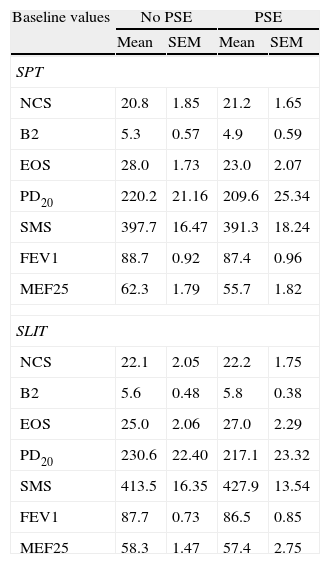
ResultsBaselineGroups at baseline did not differ for sex (Table 1A). Similarly, the age of patients at baseline did not differ between SPT and SLIT patients (respectively 12.0±0.383 and 10.8±0.524; t66=1.721, p=0.090), nor between exposed and not exposed to passive smoke (respectively 11.6±0.487 and 11.3±0.449; t66=0.444, p=0.659). The patients at baseline did not differ for their clinical parameters either (Table 1B).
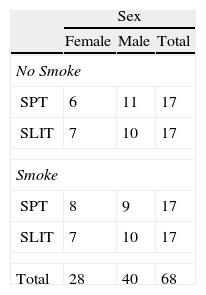
Sex ratio (A) and statistics (mean and SE of mean) (B) of the clinical parameters computed in each treatment group at baseline (SPT vs SLIT; SMEXP Smoke exposed vs Not exposed) in a study carried out from 2006 to 2008 in the Pneumology Unit of the Cuasso al Monte Hospital, Varese, Italy.
| Sex | |||
| Female | Male | Total | |
| No Smoke | |||
| SPT | 6 | 11 | 17 |
| SLIT | 7 | 10 | 17 |
| Smoke | |||
| SPT | 8 | 9 | 17 |
| SLIT | 7 | 10 | 17 |
| Total | 28 | 40 | 68 |
Chi square=0.486, df=3, PExact=0.986.
| Baseline values | No PSE | PSE | ||
| Mean | SEM | Mean | SEM | |
| SPT | ||||
| NCS | 20.8 | 1.85 | 21.2 | 1.65 |
| B2 | 5.3 | 0.57 | 4.9 | 0.59 |
| EOS | 28.0 | 1.73 | 23.0 | 2.07 |
| PD20 | 220.2 | 21.16 | 209.6 | 25.34 |
| SMS | 397.7 | 16.47 | 391.3 | 18.24 |
| FEV1 | 88.7 | 0.92 | 87.4 | 0.96 |
| MEF25 | 62.3 | 1.79 | 55.7 | 1.82 |
| SLIT | ||||
| NCS | 22.1 | 2.05 | 22.2 | 1.75 |
| B2 | 5.6 | 0.48 | 5.8 | 0.38 |
| EOS | 25.0 | 2.06 | 27.0 | 2.29 |
| PD20 | 230.6 | 22.40 | 217.1 | 23.32 |
| SMS | 413.5 | 16.35 | 427.9 | 13.54 |
| FEV1 | 87.7 | 0.73 | 86.5 | 0.85 |
| MEF25 | 58.3 | 1.47 | 57.4 | 2.75 |
SPT=Standard Pharmacological Therapy; SLIT=Sublingual Immunotherapy; NCS=Nasal Corticosteroids; B2: beta2 agonists; EOS=Eosinophils; PD20=Provocative dose that causes a 20% reduction of FEV1; FEV1=Forced expiratory volume in 1minute; MEF25=mean expiratory flow of 25%; PSE=Passive Smoke Exposure.
The preliminary analysis showed that the clinical parameters we measured at baseline (Table 1B) presented significant differences when measured after three years when the main grouping factors (treatment and smoke exposure) were considered (Table 2).
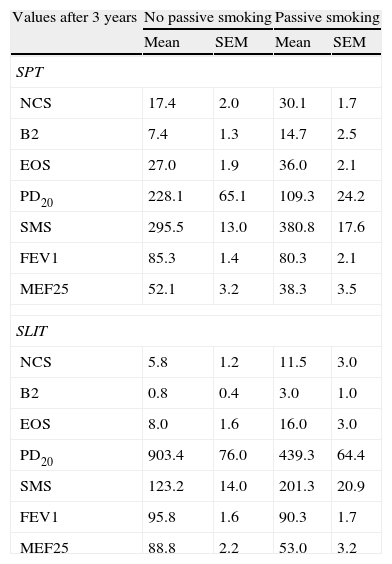
Statistics (mean and SE of mean) of the clinical parameters computed in each treatment group after three years of treatment (SPT vs SLIT; SMEXP Smoke exposed vs Not exposed) in a study carried out from 2006 to 2008 in the Pneumology Unit of the Cuasso al Monte Hospital, Varese, Italy.
| Values after 3 years | No passive smoking | Passive smoking | ||
| Mean | SEM | Mean | SEM | |
| SPT | ||||
| NCS | 17.4 | 2.0 | 30.1 | 1.7 |
| B2 | 7.4 | 1.3 | 14.7 | 2.5 |
| EOS | 27.0 | 1.9 | 36.0 | 2.1 |
| PD20 | 228.1 | 65.1 | 109.3 | 24.2 |
| SMS | 295.5 | 13.0 | 380.8 | 17.6 |
| FEV1 | 85.3 | 1.4 | 80.3 | 2.1 |
| MEF25 | 52.1 | 3.2 | 38.3 | 3.5 |
| SLIT | ||||
| NCS | 5.8 | 1.2 | 11.5 | 3.0 |
| B2 | 0.8 | 0.4 | 3.0 | 1.0 |
| EOS | 8.0 | 1.6 | 16.0 | 3.0 |
| PD20 | 903.4 | 76.0 | 439.3 | 64.4 |
| SMS | 123.2 | 14.0 | 201.3 | 20.9 |
| FEV1 | 95.8 | 1.6 | 90.3 | 1.7 |
| MEF25 | 88.8 | 2.2 | 53.0 | 3.2 |
SPT=Standard Pharmacological Therapy; SLIT=Sublingual Immunotherapy; NCS=Nasal Corticosteroids; B2=beta2 agonists;
EOS=Eosinophils; PD20=dose inducing a 20% reduction of FEV1, being FEV1=Forced expiratory volume in 1minute; MEF25=mean expiratory flow of 25%.
Moreover, all the clinical parameters showed that both the treatment factors (TREAT: SLIT vs SPT; Table 2) and the smoke exposure (SMEXP: exposed vs not exposed to passive smoke; Table 2) had significant effects in shaping both the differences observed within and between subjects (Table 3; Figs. 1–3).
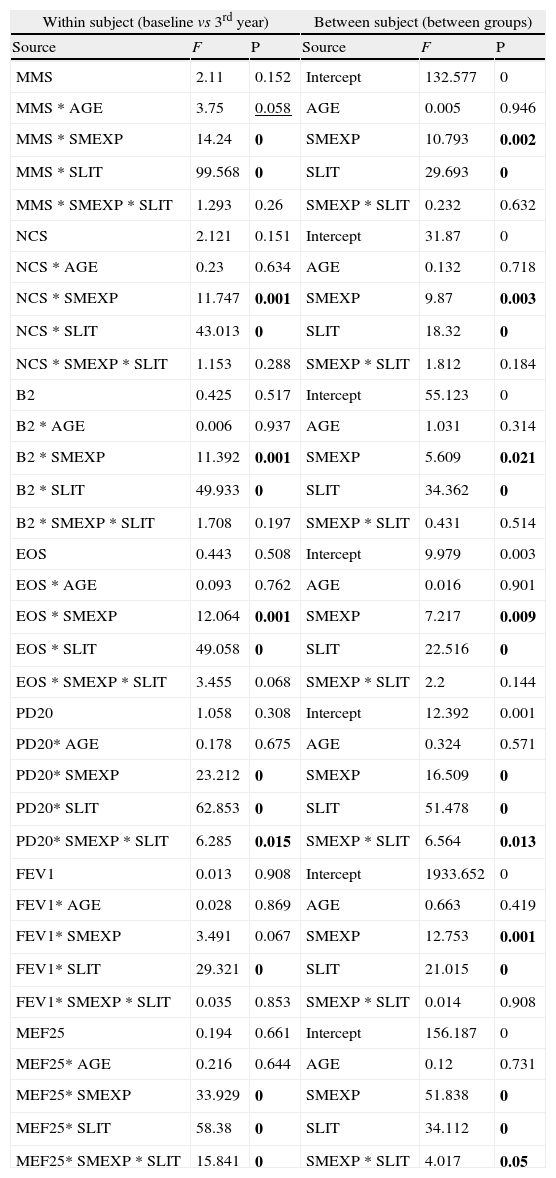
Results from multi-way ANOVA for repeated measures calculated on the clinical parameters in each treatment group (SPT vs SLIT; SMEXP Smoke exposed vs Not exposed) in a study carried out from 2006 to 2008 in the Pneumology Unit of the Cuasso al Monte Hospital, Varese, Italy. F values and significance (P) are reported for both within and between subjects tests.
| Within subject (baseline vs 3rd year) | Between subject (between groups) | ||||
| Source | F | P | Source | F | P |
| MMS | 2.11 | 0.152 | Intercept | 132.577 | 0 |
| MMS * AGE | 3.75 | 0.058 | AGE | 0.005 | 0.946 |
| MMS * SMEXP | 14.24 | 0 | SMEXP | 10.793 | 0.002 |
| MMS * SLIT | 99.568 | 0 | SLIT | 29.693 | 0 |
| MMS * SMEXP * SLIT | 1.293 | 0.26 | SMEXP * SLIT | 0.232 | 0.632 |
| NCS | 2.121 | 0.151 | Intercept | 31.87 | 0 |
| NCS * AGE | 0.23 | 0.634 | AGE | 0.132 | 0.718 |
| NCS * SMEXP | 11.747 | 0.001 | SMEXP | 9.87 | 0.003 |
| NCS * SLIT | 43.013 | 0 | SLIT | 18.32 | 0 |
| NCS * SMEXP * SLIT | 1.153 | 0.288 | SMEXP * SLIT | 1.812 | 0.184 |
| B2 | 0.425 | 0.517 | Intercept | 55.123 | 0 |
| B2 * AGE | 0.006 | 0.937 | AGE | 1.031 | 0.314 |
| B2 * SMEXP | 11.392 | 0.001 | SMEXP | 5.609 | 0.021 |
| B2 * SLIT | 49.933 | 0 | SLIT | 34.362 | 0 |
| B2 * SMEXP * SLIT | 1.708 | 0.197 | SMEXP * SLIT | 0.431 | 0.514 |
| EOS | 0.443 | 0.508 | Intercept | 9.979 | 0.003 |
| EOS * AGE | 0.093 | 0.762 | AGE | 0.016 | 0.901 |
| EOS * SMEXP | 12.064 | 0.001 | SMEXP | 7.217 | 0.009 |
| EOS * SLIT | 49.058 | 0 | SLIT | 22.516 | 0 |
| EOS * SMEXP * SLIT | 3.455 | 0.068 | SMEXP * SLIT | 2.2 | 0.144 |
| PD20 | 1.058 | 0.308 | Intercept | 12.392 | 0.001 |
| PD20* AGE | 0.178 | 0.675 | AGE | 0.324 | 0.571 |
| PD20* SMEXP | 23.212 | 0 | SMEXP | 16.509 | 0 |
| PD20* SLIT | 62.853 | 0 | SLIT | 51.478 | 0 |
| PD20* SMEXP * SLIT | 6.285 | 0.015 | SMEXP * SLIT | 6.564 | 0.013 |
| FEV1 | 0.013 | 0.908 | Intercept | 1933.652 | 0 |
| FEV1* AGE | 0.028 | 0.869 | AGE | 0.663 | 0.419 |
| FEV1* SMEXP | 3.491 | 0.067 | SMEXP | 12.753 | 0.001 |
| FEV1* SLIT | 29.321 | 0 | SLIT | 21.015 | 0 |
| FEV1* SMEXP * SLIT | 0.035 | 0.853 | SMEXP * SLIT | 0.014 | 0.908 |
| MEF25 | 0.194 | 0.661 | Intercept | 156.187 | 0 |
| MEF25* AGE | 0.216 | 0.644 | AGE | 0.12 | 0.731 |
| MEF25* SMEXP | 33.929 | 0 | SMEXP | 51.838 | 0 |
| MEF25* SLIT | 58.38 | 0 | SLIT | 34.112 | 0 |
| MEF25* SMEXP * SLIT | 15.841 | 0 | SMEXP * SLIT | 4.017 | 0.05 |
Bold: P<0.050; Underlined: P>0.050 and P<0.060
MMS=mean monthly symptoms scores; SMEXP Smoke exposure; SLIT=Sublingual Immunotherapy treatment; NCS=Nasal Corticosteroids; B2=beta2 agonists; EOS=Eosinophils; PD20=dose inducing a 20% reduction of FEV1, being FEV1=Forced expiratory volume in 1minute; MEF25=mean expiratory flow of 25%.
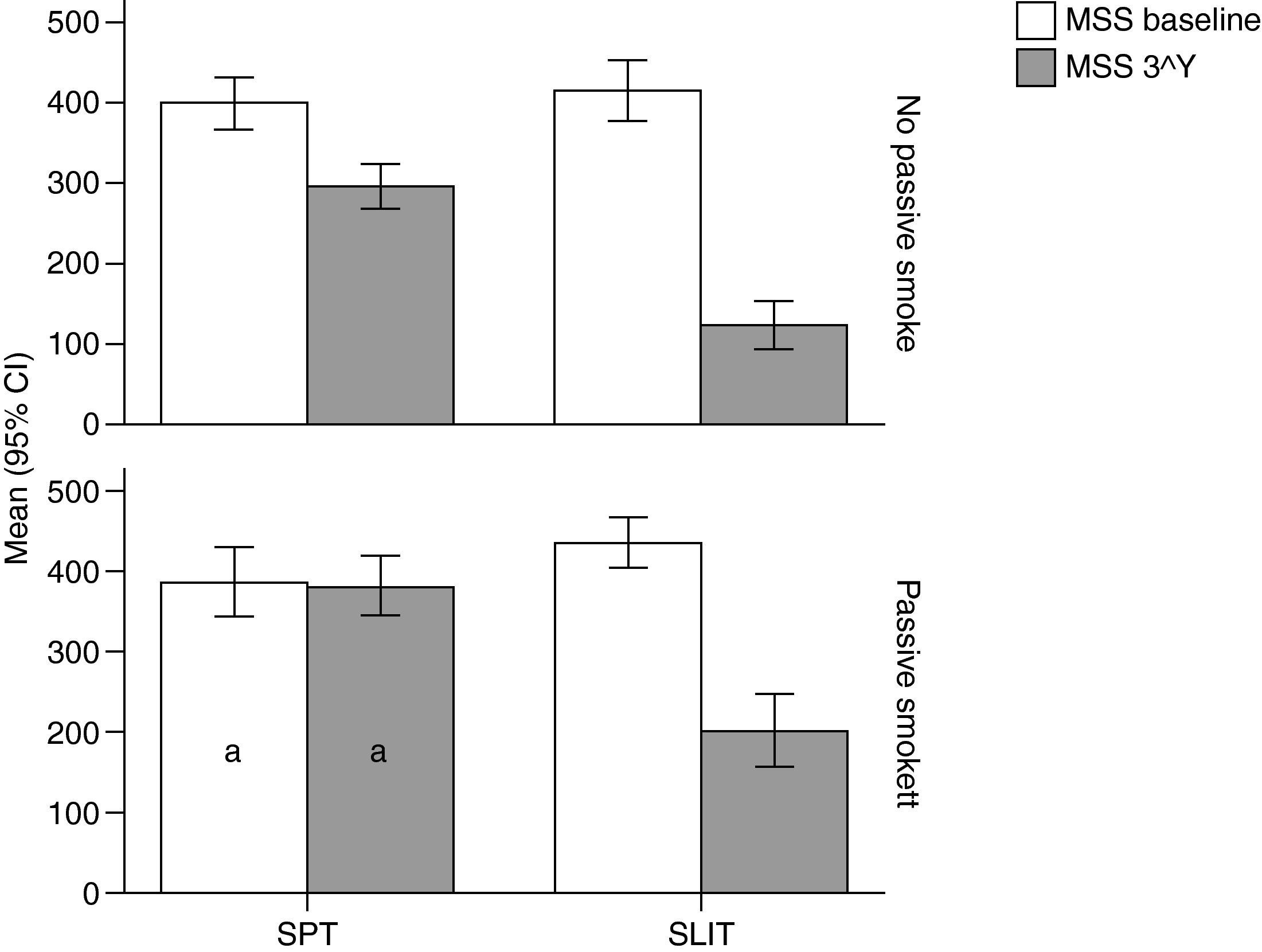
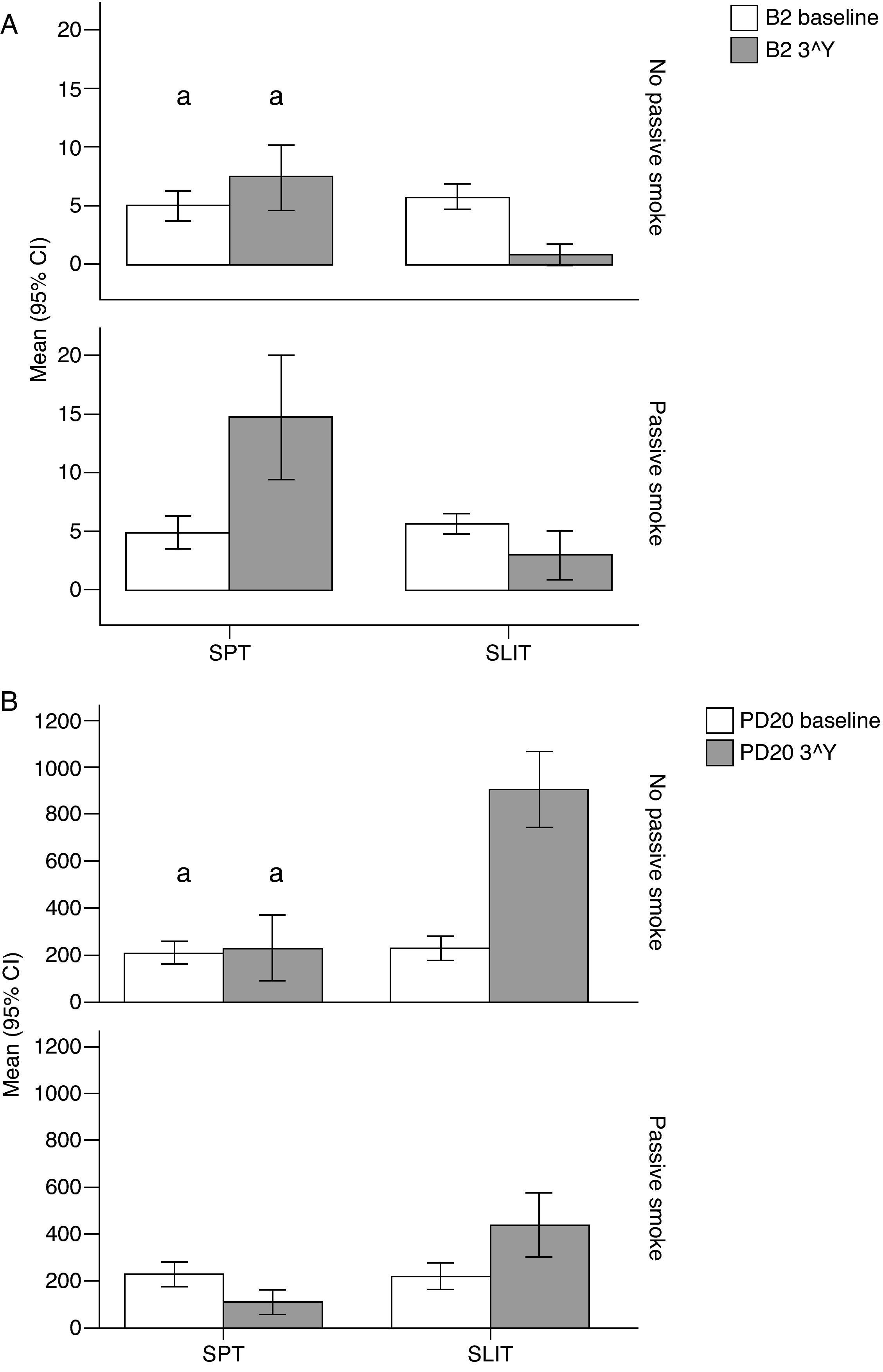
Variation (baseline vs 3rd year) of Mean Monthly Symptoms Scores (mean and 95% Confidence Intervals) in patients under different treatment conditions (SPT [Standard Pharmacological Therapy] vs SLIT [SubLingual Immuno-Therapy]) and different exposure to passive smoke (Exposed vs Not exposed) in a study carried out from 2006 to 2008 in the Pneumology Unit of the Cuasso al Monte Hospital, Varese, Italy. Groups which did not present significant differences are highlighted with the same letters.
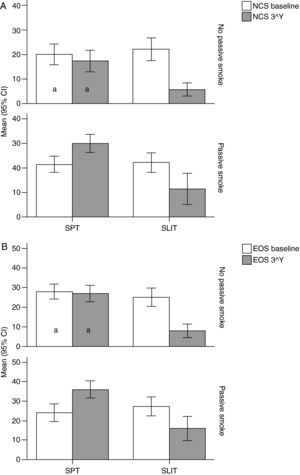
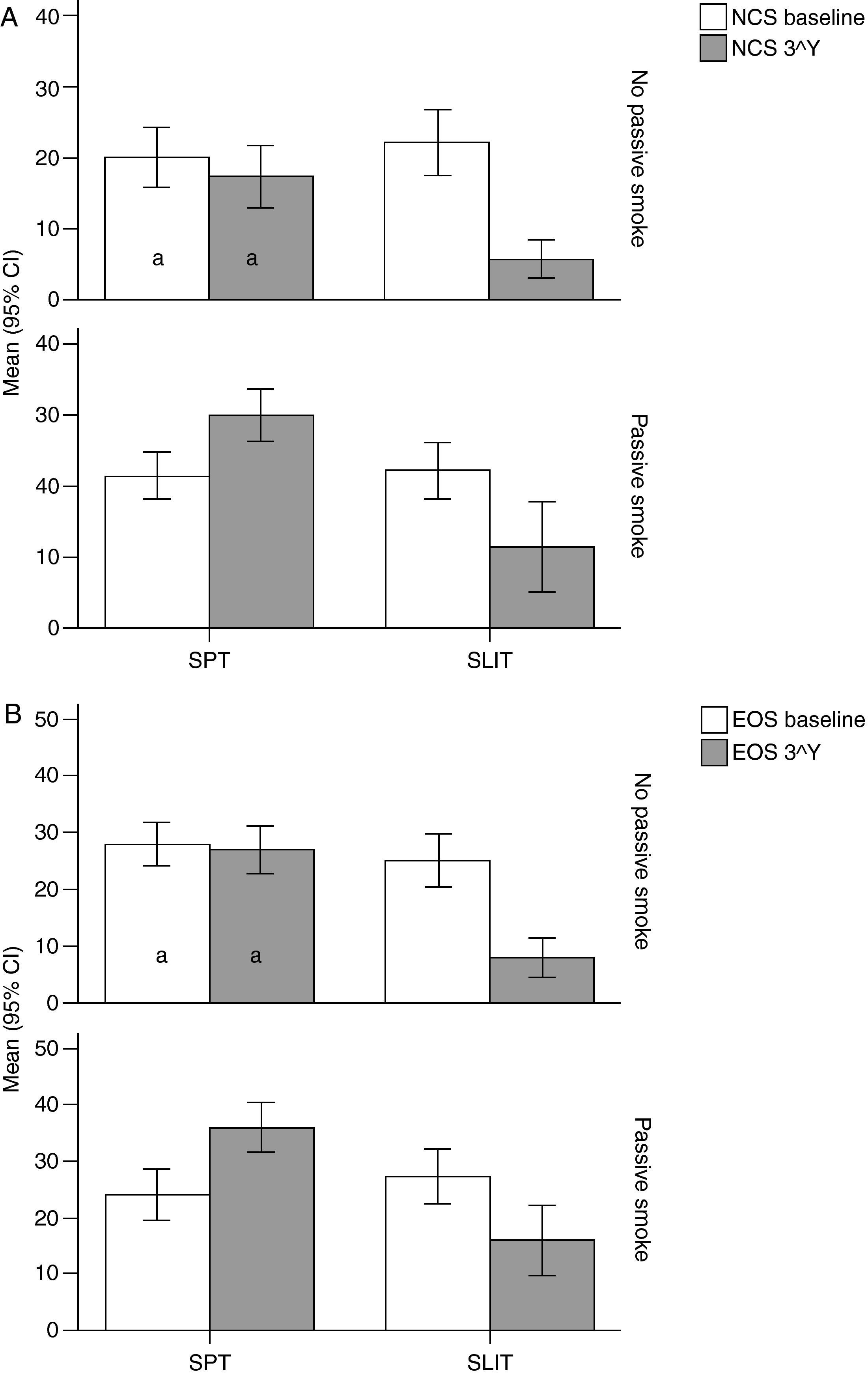
Variation (baseline vs 3rd year) of Nasal Corticosteroids (NCS), and B) Eosinophils numbers (EOG) values (mean and 95% Confidence Intervals) in patients under different treatment conditions (SPT Standard Pharmacological Therapy vs SLIT (SubLingual Immuno-Therapy) and different exposure to passive smoke (Exposed vs Not exposed) in a study carried out from 2006 to 2008 in the Pneumology Unit of the Cuasso al Monte Hospital, Varese, Italy. Groups which did not present significant differences are highlighted with the same letters.
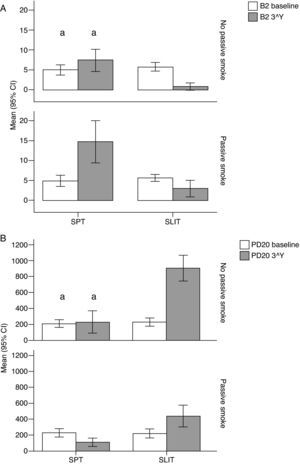
Variation (baseline vs 3rd year) of A) Beta-2 (B2), and B) Positive MCh challenge for PD20FEV1 (PD20) values (mean and 95% Confidence Intervals) in patients under different treatment conditions (SPT Standard Pharmacological Therapy vs. SLIT SubLingual Immuno-Therapy) and different exposure to passive smoke (Exposed vs Not exposed) in a study carried out from 2006 to 2008 in the Pneumology Unit of the Cuasso al Monte Hospital, Varese, Italy. Groups which did not present significant differences are highlighted with the same letters.
The overall mean monthly symptoms scores (MMS; Fig. 1) for each patient at baseline showed a significant change at the third year with a clear positive influence of SLIT treatment and negative of the exposure to smoke (TREAT Within: F1,56=43.013, p<0.001; SMEXP Within: F1,56=11.747, p=0.001). A similar pattern was detected when we compared the differences between the groups of patients treated and exposed to passive smoke (TREAT Between: F1,56=18.320, p<0.001; SMEXP Between: F1,56=9.870, p=0.003). The PD20 showed a huge increase in the group of SLIT patients who were not exposed to passive smoke (Fig. 3B).
Interestingly, the exposure to passive smoke influenced the efficacy of the STP significantly and dramatically, nullifying its effects (Fig. 1), or even worsening the overall clinical conditions at baseline.
The smoke exposure also had a negative effect on the pulmonary capacity (FEV1 and MEF25) of the patients treated with SLIT as well, although it did not exert a worsening of the clinical conditions.
New sensitisationsThe logistic model (Hosmer and Lemeshow Test: Chi-square=5.036, df=7, p=0.656) showed that the gender (SEX), the age (AGE), and the treatment (TREAT) determined the occurrence of new sensitisations, but no significant effect of passive smoke was detected.
DiscussionMany observational studies conducted over the last few years suggest that exposure to passive tobacco smoke both in the uterus and during the neonatal and infancy stages negatively influences pulmonary function, predisposing children to bronchial hyperreactivity and bronchial asthma, but without playing a significant role in predisposition to an allergic constitution (i.e. to atopy).14,15 Similarly, a genetic predisposition (heredity of asthma) together with the early onset of an atopic constitution during the first few years of life, in addition to exposure to passive smoke and recurrent respiratory infections, are a decisive negative prognostic factor in the onset of bronchial asthma in children during their transition from infancy into adolescence.16 Therefore, although a specific genotypic profile associated with environmental risk factors (e.g. passive smoke and viral respiratory infections) appears to be correlated with a greater incidence of infantile bronchial asthma, the nature of this association is still uncertain, in the light of current knowledge.17 Breastfeeding appears to lower the risk of bronchial asthma, especially if continued beyond six months, even in children exposed to maternal passive smoke. In this case too, the defensive mechanisms are unknown, although a greater immunological effect against viral infections and a delay in the onset of an atopic constitution probably come into play.18 So the roles of risks and defensive factors with respect to bronchial asthma are still far from being disentangled, and even more so, how passive smoke interferes with the treatment of bronchial asthma.
This prospective study determined to what extent the exposure to passive cigarette smoke reduces the therapeutic effect of sublingual immunotherapy and the standard drug therapy used to treat respiratory allergic diseases. Even if the patients exposed to passive smoke did not demonstrate the development of new sensitisations, in accordance with literature,15,16 the chronic mucosal phlogosis caused by combustion products (oxidants) can perpetuate the persistent immunophlogosis due to HDM. This increases the eosinophilic phlogosis, which in our study resulted to be more severe in patients exposed to passive smoke. Clinically, this leads to a worsened symptomatic profile and thus to a greater use of symptomatic therapies. Moreover, it worsens the respiratory functional indices and lowers the threshold of aspecific bronchoreactivity, with a greater incidence of persistent asthma after three years. There is no doubt that sublingual immunotherapy which acts by eliminating the eosinophilic mucosal inflammation,19 is beneficial, even though its effects are reduced in passive smoking patients.
In conclusion, this study shows that the exposure to passive smoke by children with respiratory allergy due to house dust mites lowers or nullifies the clinical response to standard drug therapy and, reduces the efficacy of sublingual immunotherapy which still exerts an overall positive significant clinical response.
Conflict of interestThe authors have no conflict of interest to declare.




![Variation (baseline vs 3rd year) of Mean Monthly Symptoms Scores (mean and 95% Confidence Intervals) in patients under different treatment conditions (SPT [Standard Pharmacological Therapy] vs SLIT [SubLingual Immuno-Therapy]) and different exposure to passive smoke (Exposed vs Not exposed) in a study carried out from 2006 to 2008 in the Pneumology Unit of the Cuasso al Monte Hospital, Varese, Italy. Groups which did not present significant differences are highlighted with the same letters. Variation (baseline vs 3rd year) of Mean Monthly Symptoms Scores (mean and 95% Confidence Intervals) in patients under different treatment conditions (SPT [Standard Pharmacological Therapy] vs SLIT [SubLingual Immuno-Therapy]) and different exposure to passive smoke (Exposed vs Not exposed) in a study carried out from 2006 to 2008 in the Pneumology Unit of the Cuasso al Monte Hospital, Varese, Italy. Groups which did not present significant differences are highlighted with the same letters.](https://static.elsevier.es/multimedia/03010546/0000003900000002/v1_201304101045/S0301054610002648/v1_201304101045/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)