Eosinophilic esophagitis (EoE) is frequently miss-diagnosed or overlooked for several years because of the invasiveness of investigations and the non-specificity of symptoms in childhood. Due to the lack of specific recommendations in children, its management remains very heterogeneous, especially concerning allergy testing. The aim of this study is to analyze our population and practices, in comparison with the literature, with a focus on allergic management, to harmonize and optimize our practice.
Material and methodsWe included all children with a diagnosis of EoE at the Hospital Femme Mere Enfant, Bron, France. Data were collected via retrospective chart review.
Results108 patients were included with an average age of 9.5 years. Average delay before diagnosis was 6.65 years. Symptoms varied with age, with a predominance of vomiting (60% of patients), feeding difficulties (72%) and growth difficulties (24%) in children <5 years, whereas older children often presented with feeding blockage (64%) and dysphagia (61%). Cough was frequent in our cohort (18.5%), especially in children <10 years (38.5% between three and five years). The allergic background was frequent (70.3%) and 80% of our patients benefited from allergy testing. Allergy testing was particularly useful to guide therapy as elimination diet represented an effective treatment in 60% of our patients
ConclusionsAllergy testing has to be harmonized to include major allergens (egg, milk, peanut, fish, wheat, and soy), including prick and patch tests. Allergy-testing based diet seemed to be the best compromise between efficiency and constraints, especially in mono-sensitized patients.
Eosinophilic esophagitis (EoE) is a chronic immune-mediated disease with an incidence rising over time, estimated at 1/2500/year, which affects children or young adults.1 Clinical presentation, related to esophageal dysfunction, varies with age: non-specific symptoms (feeding difficulties, vomiting, failure to thrive…) in children under five years old, whereas older children often relate dysphagia or food impaction. Histologically, pathology found an esophageal infiltration by eosinophils, with a cluster at 15 eosinophils/high-power-field (HPF).2 EoE is often associated with atopic diseases and food allergies seems to be involved.3 Thus, since 2007, guidelines recommended allergy testing of these patients.2 However, the utility of allergy testing is still debated and not yet codified. Moreover, invasiveness of endoscopy limits management in children which is not well determined, emphasizing the need for harmonization. To optimize our practices, we analyzed our pediatric population and its management, with a focus on allergy assessment, in comparison with the literature.
MethodsThis is a retrospective review of charts of children, from 0 to 18 years of age, with a diagnosis of EoE from January 1, 2006 to January 31, 2016 in our pediatric center (Hôpital Femme Mère Enfant, Bron, France). EoE was defined as ≥15 eosinophils/HPF (High-Power Field) in at least one esophageal biopsy. The study was approved by the local review board. A p-value <0.05 was considered as statistically significant.
ResultsDemographic and clinical characteristicsWe included 108 children: 86 boys (79.6%) and 22 girls (20.3%, ratio men/women 3.9). Average age at diagnosis was 9.5 years (from nine months to 17 years). Atopic personal background was present in 76 children (70.3%), including asthma (61.8%), food allergy (54%), allergic rhinitis (51%) and atopic dermatitis (39%). Familial atopy background was notified in 46.3% of patients. Median follow up was 5.4 years. A total of 201 endoscopies was realized during the study period.
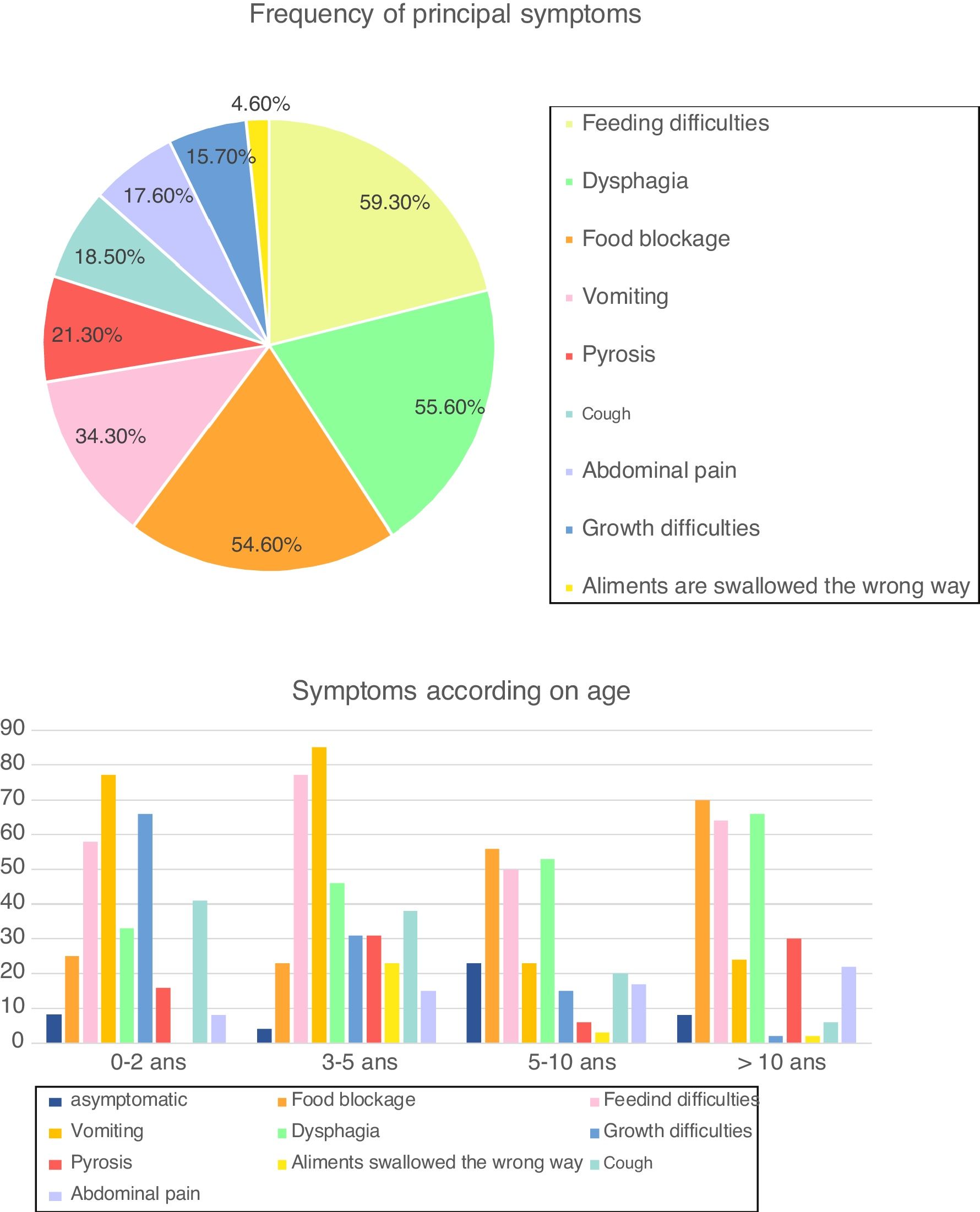
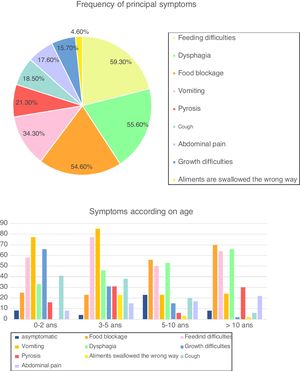
SymptomsAt the time of diagnosis, 94 patients (87.1%) were symptomatic, while the diagnosis of EoE was incidental in 14 children (12.9%). Symptoms varied with age, with a predominance of vomiting (73.0% of patients), feeding difficulties (65.3%) and growth difficulties (46.1%) in children <5 years, whereas older children often presented with food blockage (64.6%) and dysphagia (60.9%) (Fig. 1). Cough was frequent in our cohort (18.5%), especially in children <10 years (38.5% of children between three and five years and 20.6% between five and 10 years).
Frequency of symptoms and according to age (in percentage) (n=108). Fig. 1 describes frequency of principal symptoms in our total cohort and in sub-groups, according to age. In the global population, we found a predominance of feeding difficulties (59.3% of our cohort), dysphagia (55.6%), food blockage (54.6%) and vomiting (34.4%), followed by (in order) pyrosis, cough, abdominal pain, growth difficulties and foods swallowed in the wrong way.
In sub-groups analysis, we reported a predominance of with a predominance of vomiting (73.0% of patients), feeding difficulties (65.3%) and growth difficulties (46.1%) in children <5 years, whereas older children often presented with food blockage (64.6%) and dysphagia (60.9%).
Median delay until endoscopy was 6.65 years after first symptoms (from two months to 15 years). Endoscopy was performed with a strong conviction of EoE in 78 patients (72.2%), in presence of evocating symptoms (68/78) or acute blockage (10/78). In four cases (3.7%), the diagnosis could be evocated because of esophageal dilatation (one patient), digestive troubles (two patients) or gastroesophageal reflux (one patient). In the 26 other cases (24.0%), the clinical picture did not point to EoE and endoscopy was proposed in another context.
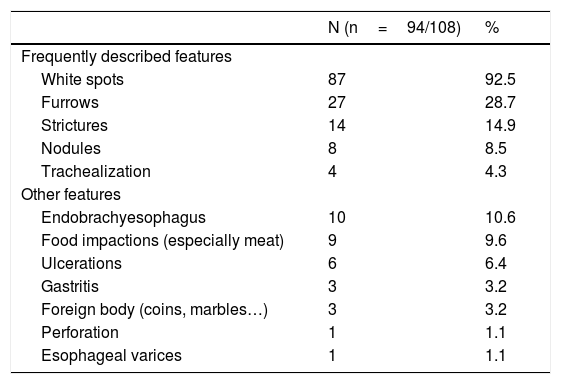
Endoscopy was macroscopically abnormal in 94 patients (87%). Principal abnormalities included white spots (92.5%), furrows (28.7%) and strictures (14.9%) (Table 1).
Endoscopic features in our cohort of patients with EoE. Endoscopic features in initial endoscopy in our cohort. We found similar reports than in the literature, with the presence of white spots in 92.5% of our patients, furrows in 28.7% and strictures in 14.9%. Nodules and trachealization were less frequent.
| N (n=94/108) | % | |
|---|---|---|
| Frequently described features | ||
| White spots | 87 | 92.5 |
| Furrows | 27 | 28.7 |
| Strictures | 14 | 14.9 |
| Nodules | 8 | 8.5 |
| Trachealization | 4 | 4.3 |
| Other features | ||
| Endobrachyesophagus | 10 | 10.6 |
| Food impactions (especially meat) | 9 | 9.6 |
| Ulcerations | 6 | 6.4 |
| Gastritis | 3 | 3.2 |
| Foreign body (coins, marbles…) | 3 | 3.2 |
| Perforation | 1 | 1.1 |
| Esophageal varices | 1 | 1.1 |
14 patients (13 %) presented a normal upper endoscopy examination but histological EoE.
On average, children had 13.8 biopsies per endoscopy (from 2 to 31). 92 patients (85.2%) had a specimen of the three parts of the esophagus.
Eosinophil density ranged from 15 to more than 100 eosinophils/HPF. Among the patients, 103 (95.4%) had at least one sign of chronic esophagitis (increased height of papillary ridges, lamina propria fibrosis, basal cell hyperplasia) and 82 patients (75.9%) had eosinophilic micro-abscesses.
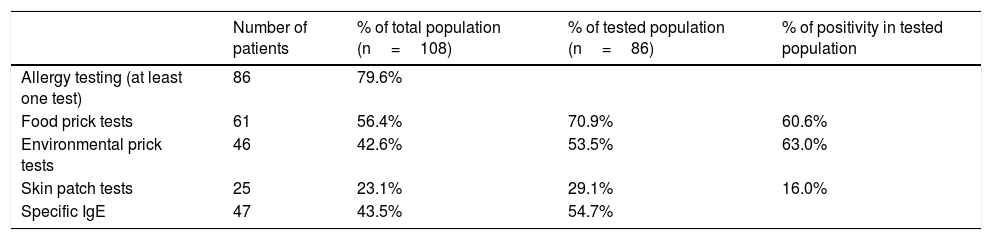
Allergen sensitization profileIn our population, 86 patients (79.6%) benefited from allergy testing (Table 2).
Repartition of allergy testing. Table 2 describes allergy testing in our cohort, with percentage of patients who benefited from food prick tests, environmental prick tests, skin patch tests, and specific IgE levels. Thus, a majority of our cohort benefited from allergy testing, which was frequently positive.
| Number of patients | % of total population (n=108) | % of tested population (n=86) | % of positivity in tested population | |
|---|---|---|---|---|
| Allergy testing (at least one test) | 86 | 79.6% | ||
| Food prick tests | 61 | 56.4% | 70.9% | 60.6% |
| Environmental prick tests | 46 | 42.6% | 53.5% | 63.0% |
| Skin patch tests | 25 | 23.1% | 29.1% | 16.0% |
| Specific IgE | 47 | 43.5% | 54.7% |
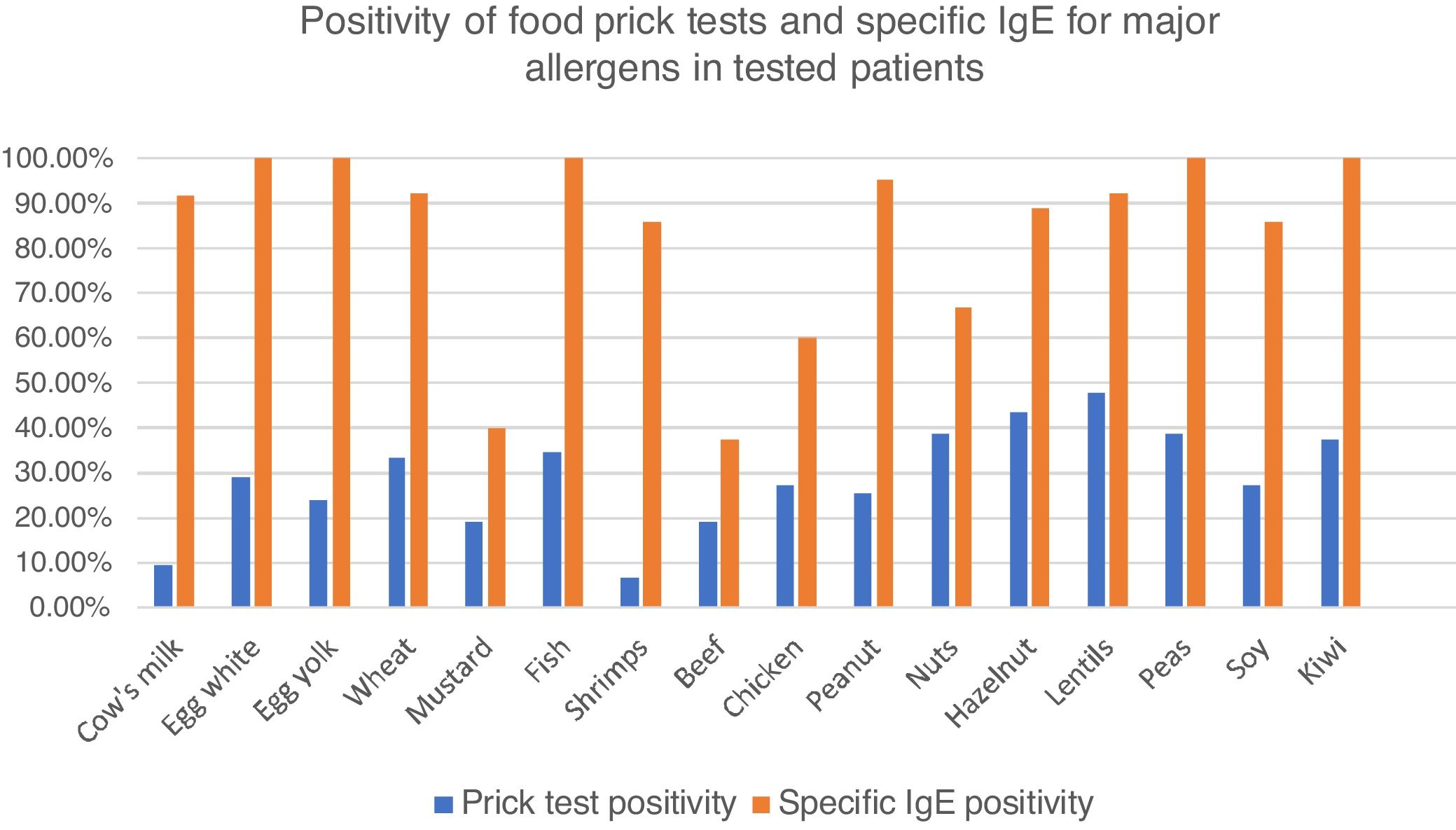
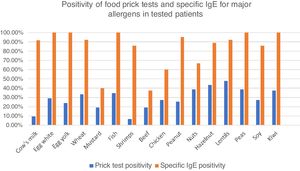
Among these patients, 77.2% had food skin prick tests, with at least one positive test in 60.7%. Principal identified allergens were lentils (47.5% of tested patients), nuts (38.5%) fish (34.6%), wheat (33.3%), eggs (28.9%), soy and chicken (27.3%) and peanuts (25.6%). Cow’s milk was positive in only 9.3% of tested patients. (Fig. 2).
Percentages of positive food prick tests for major allergens. Fig. 2 represents percentage of positive food prick tests and specific IgE for major allergens. We found similar allergens than in the literature but also high positivity for meats, especially chicken. IgE was clearly more frequently positive than prick tests.
Atopy patch tests were performed in 25 patients (31.6%) and were positive in four cases (16%): peanut (1/4), wheat and peanut (1/4), egg (1/4) and milk (1/4). The severity of reaction after SPT was not correlated to the diameter of the prick test. Atopy patch tests were concordant with prick tests in three patients (75%).
Specific IgE blood levels were measured in 47 patients (54.7%). The principal identified allergens were eggs and fish (100% of tested patients), peanuts (95.0%), lentils and wheat (92.3%) cow’s milk (91.7%), soy (85.7%), nuts (66.7–88.9 %) and chicken (60.0%) (Fig. 2).
ISAC (Immuno Solid-phase Allergen Chip) was performed in 29 patients (26.8%), and positive in 24/28 (82.7%). Frequent antigens found were (in order) soy, kiwi, peanut, egg, milk, and fish. In 10 cases, allergen families were identified (PR 10, LTP and profilin).
By combining these tests, we identified 34 patients (39.5%) without sensitization for food allergens, eight mono-sensitized patients (9.3%), 10 patients sensitized to two groups of food allergens (11%), and 34 patients sensitized to ≥3 groups (39.5%).
Environmental sensitization was very frequent in our population with at least one positive test in 63.0%. Sensitization for environmental allergens was statistically more frequent in patients sensitized for several groups of food allergens.
ManagementProton Pump Inhibitors (PPI) therapy permitted amelioration of symptoms in 71.5% of the 69 treated patients. Forty-six patients (38.9%) were treated with topical steroids (with predominance of swallowed puff of Fluticasone) with clinical efficacity in 88.9%.
Among other treatments, systemic steroids were used in 11 patients (11.1%), anti-histamine in 17 patients (15.7%) and Omalizumab in two patients. Esophageal dilation was performed in three cases (2.8%).
Forty patients (37.0%) benefited from diets: spontaneous incriminated food elimination was used in seven patients (17.5%), a diet based on allergy test in 28 patients (70.0%), protein hydrolysate formula in one patient (2.5%) and amino acid formula in four patients (10.0%). None of our patients followed 6-Food Elimination Diet. The diet duration ranged from 2 to 24 months. Clinical and histological efficiency were reported in 60% and 35% of patients respectively.
EvolutionAmong our 55 patients with endoscopic control, we found a disappearance of clinical and pathological signs in 13 cases (23.6%), a global improvement in 10 cases (18.1%), an improvement of only histological signs in six cases (10.9%) and only clinical symptoms in 11 cases (20.0%) Thus, evolution was favorable (remission, global or partial improvement) in 40 patients (72.7%) and unfavorable in 15 patients: persistence of both symptoms in 14 cases (25.4%) and amplification in one case (1.8%). Among them, seven patients (46.7%) belonged to the non-tested group and four other patients (26.6%) were sensitized to more than three groups of allergens.
We reported two gastric ulcers in a patient treated with swallowed steroids (1,85%). No other complication has been reported.
DiscussionDemographically, our population was consistent with the literature with a predominance of males (79.6% in our cohort versus 70–85% in literature) and young children (median age was nine years versus 5–10 years in literature)4,5 with a frequent atopic background (70.3%). As reported in previous works, familial atopic background was reported in 46.3% of our patients. Genetic variation was analyzed in literature, and some clusters were recently identified.6 Other associations have been reported (esophageal atresia, transplantation, EBV infection, preterm babies…) but the results were not significant in our cohort, perhaps due to the small sample size.
First of all, as observed in literature, symptoms in our cohort differed according to the age: child under five years presented with reflux symptoms, vomiting, dysphagia, feeding difficulty and failure to thrive, whereas older children and adolescents more likely presented with food impaction, abdominal pain and dysphagia.7,8 These two patterns of symptoms could be linked to the natural course, with a first inflammatory phase followed by fibrosis.9 Cough is rarely reported in the literature but appeared as a frequent symptom in our cohort, especially between three and 10 years and should be considered as a suggestive symptom of EoE in young children.
The delay in diagnosis (median of 6.65 years in our cohort) was consistent with literature (six years10) and can be explained by the non-specificity of symptoms, an underestimation by the patient himself, the invasive character of explorations and the overlap with gastroesophageal reflux disease. Moreover, this diagnosis could be miss diagnosed due to a normal aspect in endoscopy (20% and 7% of patients in retrospective prospective analysis, respectively11) and focal infiltration requiring multiple biopsies.12 Thus, the diagnostic sensitivity of a single biopsy was 73% in children and increased above 97% after five biopsies, so guidelines recommend at least six biopsies, including the three levels of esophagus.2 The majority of our patients benefited from these multiple biopsies, whereas endoscopy was normal in 12.9%. An endoscopic score, called EREFS, was proposed to classify and grade EoE, with great specificity (90–95%) but with low sensitivity and negative predictive value of endoscopic features (20–30%).13
The cut-off of 15 eosinophils/HPF was arbitrarily determined in 2007 guidelines, and then demonstrated 100% sensitivity and 96% specificity.14 Other signs in pathology included micro-abscesses, basal zone hyperplasia, dilated intercellular spaces, eosinophil surface layering, papillary elongation, and lamina propria fibrosis. A large majority (95.4%) of our patients presented with these other pathological signs.
Currently, diagnosis and follow up of disease activity relies on endoscopy, involving anesthesia (with a potential risk of general anesthesia on cerebral development15) and invasive methods. This can be an obstacle in the management of young children. However, none of the other exams (esophagram, manometry, EndoFLIP, biomarkers…) were actually valuable.
Clinical scores could be interesting to predict and follow EoE but dissociation between symptoms and endoscopic/histologic features limits their utilization.16 In our cohort, 13% of the patients were asymptomatic but with positive pathology.
In our center, allergy testing was a central element to guide the management of patients, including atopy patch tests, skin prick tests and IgE levels. Our frequent food allergens are concordant with literature, adding chicken, with prick tests positivity in 27.3% and IgE in 60.0% in our cohort.
Skin prick tests demonstrated a low positive predictive value (average of 47%) but a really better negative one (>90%).17 Thus, the principal goal of skin prick tests is not to determine the culprit antigen but to determine non-implicated ones to limit diet exclusions and their impact on growth and deficiencies. This is particularly important in EoE because major allergens (milk, wheat, egg, fish, nuts, peanuts and soy…) represent an important part of a child’s diet.
The use and interpretation of atopy patch tests are not standardized in EoE because concordance between patch test results and EoE’s food triggers identified by biopsy-monitored sequential food reintroduction is low, with <50% sensitivity, except for milk (86%). However, they are frequently used in pediatric cohorts where results are better than in adults with a positive predictive >90%. Thus, in our experience, atopy patch tests for milk, wheat, potatoes, egg, beef, and chicken proved their benefit and have the advantage of testing non-immediate allergic reactions (physiopathology of EoE staying unclear with potential limited relevance of IgE). Moreover, the combination of prick and atopy patch tests increases the sensitivity rate to 65–95% and Henderson et al. found that combination to be effective to guide therapy.18 Thus, we tend to generalize the use of atopy patch tests.
As for the prick test, the negative predictive value of IgE testing in better than the positive one but concordance with symptoms is low.19 Several studies showed that patients with low or undetectable IgE could respond to the eviction of incriminated food. Thus, recombinant humanized anti-IgE antibody (Omalizumab) seems ineffective to treat EoE.20 Notwithstanding, IgE can be useful to identify culprit allergens in case of a significant increase.
Chip was evaluated to guide diet and effective in 66.7% of our patients but the literature results are not convincing, with 93% of patients who failed to achieve endoscopic remission.21 However, these results concern an adult population and results of allergy testing are different between an adult and a pediatric cohort.
Other tests (MAST CLA, serum IgE levels, blood eosinophils count…) described in our cohort were not recommended in guideline because of non-encouraging results. We found no relation between blood eosinophilic count and esophageal eosinophilic count.
Therefore, allergy testing cannot affirm the diagnosis but could sometimes help to identify foods and undoubtedly guide dietary therapy to resolve symptoms and avoid unnecessary exclusions (to limit the impact on growth and deficiencies but also limit the risk of subsequent anaphylaxis in case of prolongated exclusion in sensitized allergens).
The dietary approach is sustained by the efficacy of amino acid-based formula in resolving the disease with a remission rate of 90.8% but its implementation is difficult because of poor taste, social impact, cost… Therefore, some authors suggest the use of elemental diets only after failure of other diets. This diet was used in only 4.7% of our cohort. Empiric diets eliminate common food allergens without prior allergy testing. Most common are 1, 2 or 6-Food Elimination Diets, including most common allergens (milk, wheat, egg, soy, peanut and nuts, and shellfish and fish), with histological remission in 65–77%.22,23 Most children’s EoE is triggered by one to three foods, so better management would be a sequential reintroduction and monitoring of symptoms and biopsy after each change, but the necessity of repeated endoscopy limits their utilization (thus, none of our patient followed these diets).
As demonstrated by Spergel, the dietary restriction based on prick and patch tests seems to be useful, and has the advantage of excluding fewer foods.24 However, it demonstrates some limits, especially a poor predictive positive value, with clinical and histological response in only 48% of child.25 This type of diet seems to be especially effective in the mono-sensitized patient.
The goal of treatment is the resolution of inflammation to prevent disease relapse and long-term complications such as fibrosis or strictures. Several forms of therapy are currently used in practice, in addition to dietary eviction or no.26
Medical treatment includes PPIs (representing now the first line of treatment, with histologic remission and symptom improvement in 50 and 60%, respectively (71.5% in our cohort))2,27,28 and topical steroid (with equivalent efficacy and fewer adverse events than systemic corticosteroid). Fluticasone propionate swallowing, and recently viscous budesonide induced histological remission in 50–90% of patients.2,29 However, the clinical response is less clear and two recent meta-analyses did not find superiority to placebo.30
Other treatments, such as immunosuppressive treatment, Omalizumab… were not used in practice.
In our study, only 2.8% of patient needed esophageal dilation. Risks are poor if dilatation is progressive but if a clinical improvement at one year is noticed in 85% of the patients, the benefit is not effective in the long-term.
A favorable evolution was found in 72.7% of our patients, especially among mono-sensitized patients and the allergy-testing group.
Discussion and conclusionsDue to the lack of specific recommendations in children, especially regarding allergy testing, EoE management remains very heterogeneous. Management of these children has to be standardized in pediatric centers according to adult guidelines and local practices. This report underlines some interesting facts in our population such as the frequency of chronic cough in children <10 years, advantage of allergy testing (including meat tests) with a role of atopy patch test and the need for harmonization of testing and treatment.
Conflict of interestThe authors have no conflict of interest to declare.
Funding sourceThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
We would like to thank Mr. Berthiller who performed all the statistics of this work.