Diamine oxidase (DAO) is a polyamine-degrading enzyme also implicated in histamine metabolism. Chronic urticaria (CU) has a wide spectrum of clinical presentations and causes. Anisakis sensitisation associated chronic urticaria (CU+) has been characterised as a phenotype with different clinical and immunological characteristics and possibly associated with previous acute parasitism. We aimed to analyse serum DAO levels in different CU phenotypes. We further analysed the possible association of DAO with fish eating habits.
MethodsWe studied 35 CU+ patients and 39 non-sensitised CU patients (CU−) as well as 19 controls. We analysed fish-eating frequency as well as fish intake associated exacerbation of CU (FIAE) or gastro-intestinal complaints (GI). DAO levels were further analysed with respect to lymphoproliferative responses, cytokine and specific IgE production.
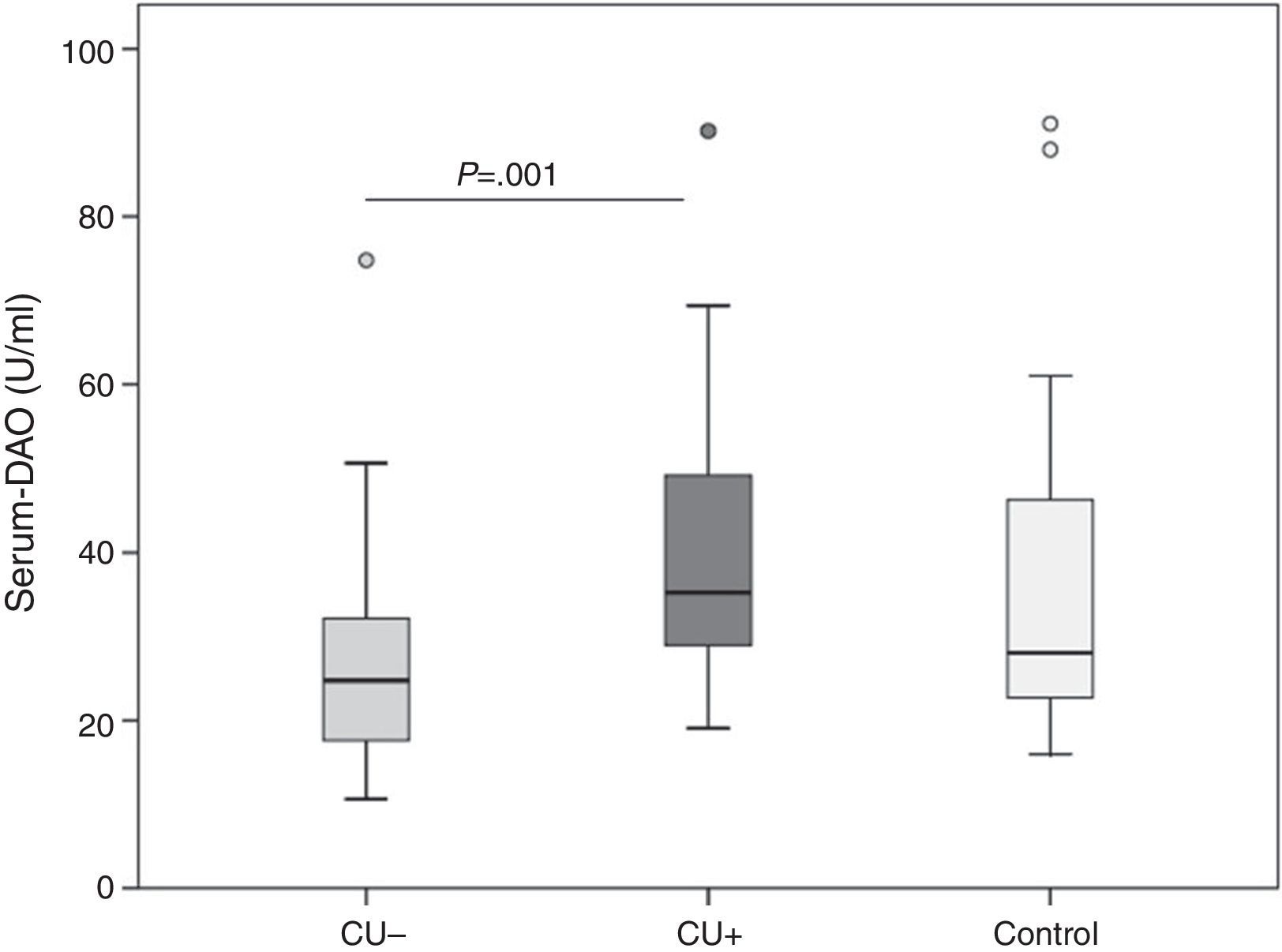
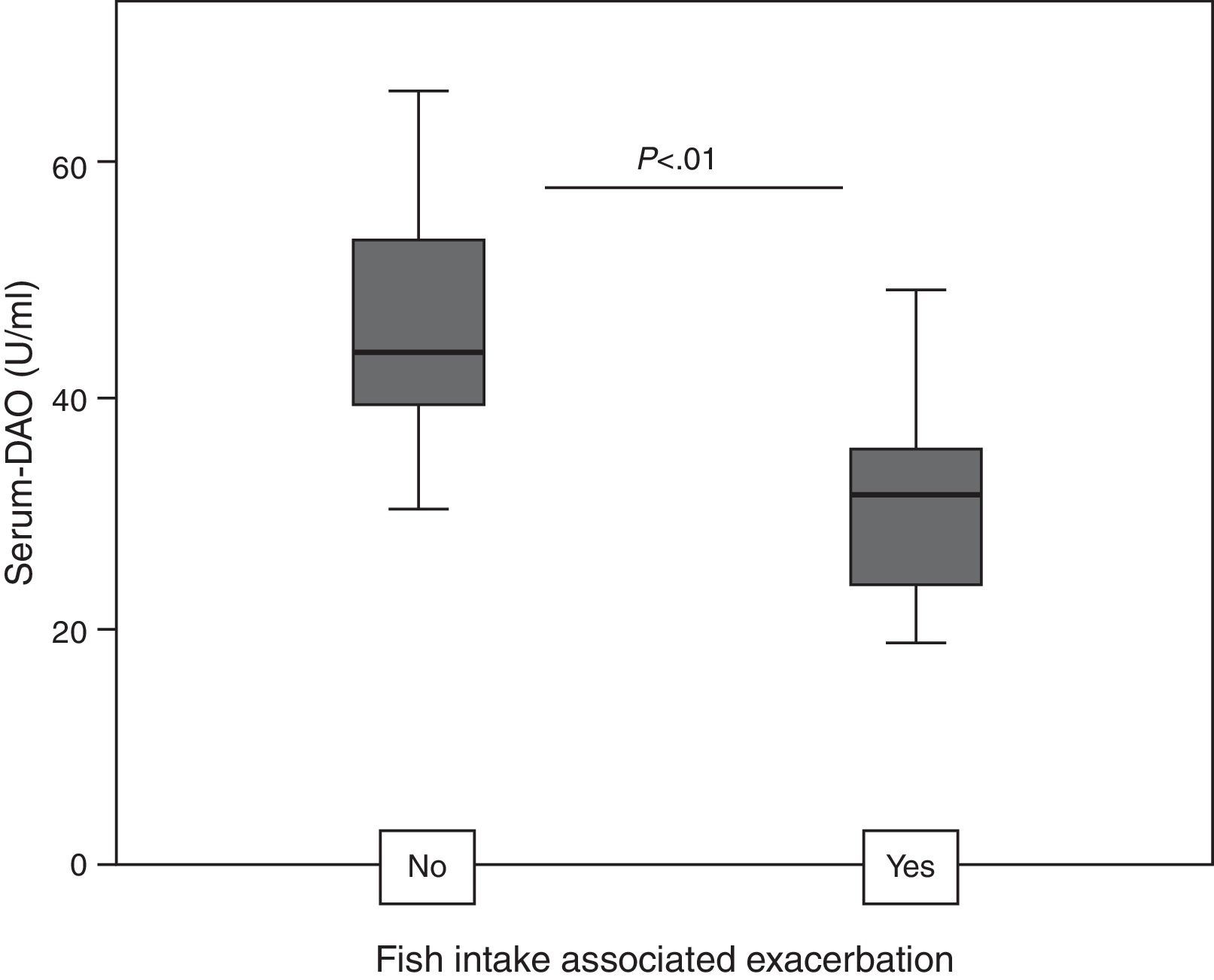
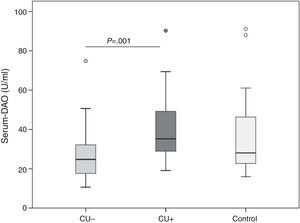
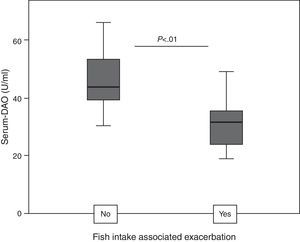
ResultsDAO levels were not different between CU and controls, but were significantly higher in CU+ than in CU−. CU+ patients with FIAE had lower DAO levels, but no differences were detected in patients with GI. DAO levels correlated positively with oily and canned fish consumption in CU−. In CU+, DAO levels correlated positively with specific Anisakis IgE, percentages of proliferation in Anisakis stimulated peripheral blood lymphocytes, serum IL-2 and IL-6, but correlated negatively with mitogen stimulated TGF-β in supernatants.
ConclusionsDAO levels in CU depend on fish-eating habits and in CU+ on the amount of specific IgE production. In the CU+ phenotype, lower levels of DAO predispose to urticaria exacerbation after fish intake, probably due to a relative insufficient enteric availability of this enzyme.
Chronic urticaria (CU) is a disease without any well-defined cause, but the clinical appearance results from the convergence of multiple possible factors on mast cell activation and degranulation, which liberates a vast array of mediators. Histamine is one of the most potent mediators and is responsible for the appearance of wheals and pruritus. Therefore, biochemical pathways in the production as well as the degradation of histamine can provide clues to the pathophysiology of this disease.
Diamine oxidase (DAO) is responsible for scavenging extracellular histamine.1 Initial studies of plasma DAO measurements were performed by displacing enteric DAO from the intestinal mucosa into the peripheral circulation by intravenous heparin administration,2 but more sensitive commercially available ELISA kits made it later possible to assess serum or plasma levels directly.
Over the last few years, DAO has gained interest in different research fields. Within the different pathologies attended at allergy clinics, the focus has been on histamine intolerance as well as on chronic urticaria. In both cases, interest is therefore directed towards possible dietary advice or substitution therapy. In the scientific literature there is still controversy on possible dietary control in CU, even though in CU about 30–40% of patients attribute their symptoms to food intolerance.3
In histamine intolerance, increased availability of biogenic amines, together with an impaired histamine degradation can lead to generalised symptoms including urticarial rash.4 Clinical parameters are the main factors leading to a suspected diagnosis, and in a high percentage of cases DAO serum levels are found to be lower than in controls.5
On the other hand, in chronic urticaria the possible implication or usefulness of DAO determination is not straightforward.3,5,6 A role of DAO in modulating biologic effects of histamine is warranted,7 but it is postulated that elevated histamine levels in CU result from secondary mediator release rather than a specific defect in the histamine metabolic pathway.8
Another feature of DAO is its possible association with intestinal gut dysfunction and is proposed as a marker of mucosal integrity.9 Thus, several human and animal studies revealed that DAO activity is inversely associated with intestinal permeability of the small intestine.10
In the Mediterranean region, Anisakis sensitisation associated chronic urticaria (CU+) is a frequent phenotype of CU.11–14 Sensitisation is due to a previous parasitic episode by Anisakis which is occasionally the eliciting factor for the chronic urticarial reaction. In CU+, patients often claim that exacerbation of urticaria is associated with fish intake. There are however reports on urticaria, but also isolated gastro-intestinal complaints after fish intake which can easily be misinterpreted as being due to an IgE-mediated response in Anisakis-sensitised patients with previous parasitism.15 A true allergic IgE-mediated cause or de novo parasitism by the fish borne nematode can only rarely be accountable for symptom worsening.16
It is known that especially oily or canned fish is rich in histidine, which is transformed to biogenic amines, such as histamine17 and thus a direct triggering of intestinal or cutaneous symptoms by fish intake could be a possible explanation, when enteric DAO is insufficiently produced. Therefore, the assessment of serum DAO could be useful in the assessment of this cause-effect relationship.
We hypothesised that in a region with high fish consumption, DAO serum levels would vary in different CU phenotypes as well as in those patients who present with adverse gastro-intestinal or urticaria symptoms after fish intake.
MethodsStudy protocolWe included prospectively 74 patients with CU: 35 of whom were sensitised against A. simplex (CU+). Nineteen subjects without a history of urticaria, adverse reactions associated with fish intake or sensitisation against A. simplex served as controls. Two phenotypes of CU were differentiated: criteria to include patients in the Anisakis sensitisation associated chronic urticaria (CU+) were a positive Skin Prick Test (SPT) against A. simplex and detectable specific IgE against Anisakis larval antigen. CU− patients had neither a positive SPT nor serum specific IgE against A. simplex. Furthermore, patients were asked for exacerbation of urticaria or gastro-intestinal complaints after fish intake. Two subgroups were established:
As fish consumption is rather high in our region and in order to reduce possible bias, fish intake associated exacerbation (FIAE), was ascertained if patients claimed at least two episodes of worsening of urticaria within six hours after fish intake. Likewise, patients claiming gastro-intestinal complaints (GI) had at least two associated episodes. A further necessary inclusion criterion in CU+ was that the implicated fish-meal (canned, deep-frozen or well-cooked fish preparation) was not suspected to have induced a new parasitic episode by Anisakis. These groups were compared with those who did not claim any fish intake associated adverse reactions. Patients with only one episode of FIAU or GI or in those situations, where a new acute parasitic episode could not be ruled out, were not included in the analysis of FIAE or GI.
Chronic urticaria was defined as wheals recurring at least twice per week for at least the previous six weeks. Patients were not included in this study if physical stimuli were the only eliciting agents of urticaria. Other known factors associated with CU, such as positive hepatitis serology, antithyroid antibodies or autoimmune status as assessed by autologous serum skin test, were not an exclusion factor, and were not further analysed.
Patients were asked for the number of weekly fish portions (fresh/deep-frozen, oily/non-oily, canned) by a standardised questionnaire as previously described.13
Urticaria activity score (UAS) was assessed as previously described.18 Shortly, severity of urticaria in CU patients was clinically assessed after withdrawing antihistamines for five days. The mean score of the last four days was calculated as the sum of the wheal number score (between 0 and 3: 0; 0–9; 10–50; >50) and the itch severity score (between 0 and 3: no; mild; moderate; severe).
The number of weekly doses of necessary antihistamines for symptom relief was asked.
This study followed the Declaration of Helsinki on medical protocol and ethics. Included patients gave written consent and the University Hospital La Princesa Ethical Committee approved the study protocol. The study was funded by grants from Fundación Sociedad Española de Alergología e Inmunología Clínica (SEAIC) and Fundación Mutua Madrileña, Spain.
Serum samples, peripheral blood mononuclear cells (PBMCs) and in vitro stimulation assayBlood was taken after antihistamines were withdrawn for five days. Serum was stored at −70°C until processing and whole blood was immediately processed for stimulation assays.
Anti-Anisakis-specific IgE was analysed by CAP-FEIA against crude extract larval antigen (Phadia, Uppsala, Sweden).
PBMCs were isolated by centrifugation over Histopaque-1077 (Sigma-Aldrich, St. Louis, MO, USA) density gradient, and cell viability was assessed by means of trypan blue dye exclusion. Cells were seeded in quadruplicate wells at 1.25×106cells/ml in RPMI 1640 supplemented with 10% heat-inactivated foetal bovine serum, 10mM HEPES buffer, 2mM l-glutamine, and 0.06g/l of gentamycin. Cells were cultured under stimulation with either Concanavalin A (ConA) from Canavalia ensiformis (Sigma-Aldrich) (50μg/ml), or A. simplex larval crude extract (500μg/ml), or with medium alone. Cells were incubated for 72h at 37°C in a humidified incubator with 5% CO2. Supernatants were stored at −70°C until further processing. Proliferative responses of ConA or Anisakis stimulated peripheral blood lymphocytes were determined by lymphoproliferative assay using MTT dye [3-(4,5-dimethyl thiazol-2-yl)-2,5-diphenyl tetrazolium bromide]. After the medium was removed by centrifugation, 20μl of MTT dye (2.5mg/ml of phosphate buffered saline (pH 7.2) stock, Sigma) was added to all the wells and the plate was incubated at 37°C in a humidified incubator with 5% CO2. After 6h, 100μl of 10% sodium dodecyl sulphate in 0.001N HCl was added to all the wells to dissolve the formazan crystals and the optical density (OD) was measured after 24h at 595nm on a microplate reader (ELX808 (Biotek). Percentage of proliferation (%P) was calculated from the formula: %P=[OD stimulated well×100/OD unstimulated well]−100.
Cytokine measurementSerum samples and supernatants of the lymphocyte stimulation assays were used for measurement of cytokine production: levels of IL-2, IL-4, IL-6, IL-10, TNF-α, IFN-γ and IL-17A were quantified using a multiplex assay in accordance with the instructions of the manufacturer (BDTM Cytometric Bead Array (CBA) Human Th1/Th2/Th17 Cytokine Kit; BD Biosciences, San Jose, CA, USA). TGF-β levels were assessed by a single plex assay (BDTM Cytometric Bead Array (CBA) Human TGF-β1 Single Plex Flex Set; BD Biosciences) as indicated by the manufacturer. All samples were analysed with BD FACSCalibur Flow cytometerTM and the results were expressed in pg/ml using the FCAP ArrayTM software.
Serum diamine oxidase determinationLevels of DAO were measured using the DAO ELISA for the in vitro determination of DAO in serum (Immundiagnostik AG, Bensheim, Germany) according to the manufacturer instructions. The assay utilises the “sandwich” technique with two polyclonal antibodies against recombinant DAO. Briefly, standards, controls, and diluted samples were added and incubated into the wells of a microplate coated with polyclonal rabbit anti-DAO antibody. Then a biotinylated polyclonal anti-DAO antibody was added into each well. In the next step, the streptavidin-peroxidase-conjugate was added and tetramethylbenzidine was used as peroxidase substrate. Finally, the reaction was stopped with an acidic solution and plates were read at 450nm. A dose response curve of OD versus standard concentration was generated, using the values obtained from the standard.
Statistical evaluationAge and UAS in the studied groups were calculated and compared by ANOVA.
Prevalences were calculated for sex in CU+ and CU− and compared by Chi-square-test.
DAO did not show a normal distribution by Kolmogoroff-Smirnoff test. Therefore, non-parametric tests and Spearman correlation coefficient were calculated for assessing the relationship between DAO with the other studied parameters.
A linear regression was modelled for analysis of multiple significant correlation results.
ResultsMean age was 41.4±16.3 in CU−, 55.3±12.2 in CU+ and 40.2±10.4 in controls.
UAS was 3.9±1.4 in CU− and 3.5±1.4 in CU− (n.s.).
72.2% of patients were female in CU− and 81.8% in CU+. No differences in DAO levels were detected between sexes (Median 30.5; Interquartile range (IQR) 23.4–41.0U/ml in female and median 33.5; IQR 19.2-45.5U/ml in male patients).
Seventeen patients had fish intake associated exacerbation (FIAE) (five in CU− and 12 in CU+) and 10 patients had no worsening of urticaria after fish intake (four in CU− and six in CU+).
Ten patients referred gastro-intestinal (GI) complaints after fish intake (four in CU− and six in CU+) and 26 had no fish intake associated gastrointestinal complaints (nine in CU− and 17 in CU+).
Patients neither associated gastrointestinal nor urticarial symptoms with typical histaminose associated food, such as chocolate, cured cheese, red wine, or strawberries.
Overall, DAO serum levels were not different in CU than in controls (median 28.1; IQR 22.4–52.4U/ml), but were higher in CU+ (35.3;IQR 28.4–49.2U/ml) than in CU− (24.8;IQR 17.5–32.3U/ml; P=0.001, Fig. 1).
Patients with FIAE had lower DAO levels than patients without FIAE, but when comparing by subgroups, this relationship was only true for CU+ (31.8; IQR 23.3–35.7 versus 43.8; IQR 37.2–56.5; P<0.01, Fig. 2).
There were no differences for median DAO levels in patients with or without GI.
UAS and the number of necessary antihistamines did not differ in the studied groups nor were these parameters associated with DAO.
DAO levels correlated positively with oily fish intake (Rho=0.39; P=0.05) and canned fish (Rho=0.54; P=0.005) consumption in CU−, but not in CU+.
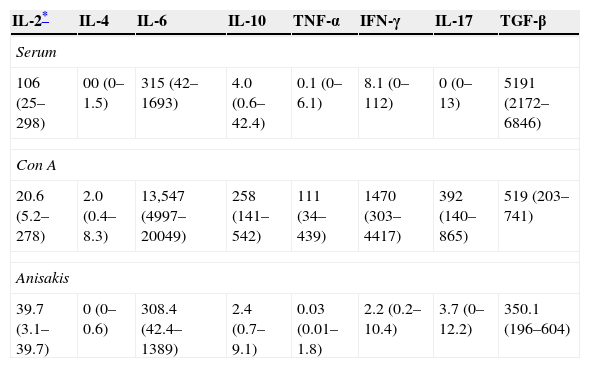
Results of cytokine measurements were available for 40 patients with CU (Table 1).
Cytokines (median and interquartile range) in serum and supernatants of Concanavalin A (Con A) or Anisakis stimulated PBMC in all studied patients. Values are in pg/ml.
| IL-2* | IL-4 | IL-6 | IL-10 | TNF-α | IFN-γ | IL-17 | TGF-β |
|---|---|---|---|---|---|---|---|
| Serum | |||||||
| 106 (25–298) | 00 (0–1.5) | 315 (42–1693) | 4.0 (0.6–42.4) | 0.1 (0–6.1) | 8.1 (0–112) | 0 (0–13) | 5191 (2172–6846) |
| Con A | |||||||
| 20.6 (5.2–278) | 2.0 (0.4–8.3) | 13,547 (4997–20049) | 258 (141–542) | 111 (34–439) | 1470 (303–4417) | 392 (140–865) | 519 (203–741) |
| Anisakis | |||||||
| 39.7 (3.1–39.7) | 0 (0–0.6) | 308.4 (42.4–1389) | 2.4 (0.7–9.1) | 0.03 (0.01–1.8) | 2.2 (0.2–10.4) | 3.7 (0–12.2) | 350.1 (196–604) |
In CU+, DAO correlated positively with Anisakis specific IgE (Rho=0.46; P<0.001).
Percentages of lymphocyte proliferation induced by in vitro Anisakis antigen stimulation correlated positively with DAO (Rho=0.76; P=0.001).
Serum IL-2 correlated with DAO levels in CU+ (Rho=0.57; P=0.03), but not in CU−. Serum IL-6 was also correlated with DAO, but reached only significance in CU+ (Rho=0.57; P=0.03).
TGF-β production induced by in vitro ConA stimulation was negatively correlated with DAO in CU+ (Rho=−0.51; P=0.04).
It was not the scope of this study to analyse cytokines in the different groups, which were published elsewhere.13 With respect to those cytokines associated significantly with DAO, only serum IL-2 differed between CU+ and CU− with higher values in CU+ (191pg/ml, IQR 58–466pg/ml) than in CU− (42pg/ml, IQR 2.2–224pg/ml; P<0.03).
We performed a linear regression model for DAO, introducing those variables which achieved P<0.05 in the correlation studies: serum IL-2 and IL-6; ConA stimulated TGF-β production; Anisakis-induced lymphocyte proliferation as well as specific IgE against Anisakis. This resulted in serum IL-2 (B=2.9; P=0.016) and IL-6 (B=2.3; P=0.047) independently explaining DAO levels.
DiscussionChronic urticaria is considered a multifactorial disease with an uncertain aetiology. It belongs to the group of chronic inflammatory conditions, which typically affect affluent countries and has frequently an autoimmune basis.19 Several hypotheses which attempt to explain the rise of inflammatory conditions are generally related to different updates or versions of the hygiene hypothesis, where dietary features and microbiota diversity and composition are crucial.20,21 Current guidelines are cautious when addressing the relationship of CU with diet. Otherwise, up to 40% of patients attribute their symptoms to foods.3 Further, several studies claim different diets to be favourable in chronic urticaria. The existing controversy could therefore be due to geographic differences and food preferences in the studied regions accounting for diverging results.
In the studied region, two epidemiological and food-associated factors contribute to the interest in performing the present study, where we assess one of the obviously important enzymes in histamine metabolism in CU. First, raw fish eating habits are associated with a high prevalence of Anisakis sensitisation associated chronic urticaria (CU+) as a differential CU phenotype, and second, overall fish consumption is very high, accounting partly for the Mediterranean diet.12,21,22
In CU, determination of DAO had not up to now demonstrated its usefulness, as their levels range within those encountered in control subjects.23 We therefore did not expect to detect pathological high or low values in CU when comparing with the expected “normal” values of our control group. We were otherwise able to observe significant and interesting results associating DAO levels with different clinical settings. We thus could demonstrate different levels both comparing CU+ versus CU− and further when assessing fish intake associated exacerbation of CU. It is known that DAO levels show a high inter-individual variability, but are otherwise relatively stable in time in one individual.l4 Therefore, even if DAO levels fall within “normal” values, and are overall not able to differentiate between CU and controls, by thoroughly analysing known factors which contribute to serum DAO levels, our results can provide new insights for the study of different urticaria phenotypes and the relationship with dietary parameters.
CU− showed lower DAO levels than CU+, and these correlate neither with UAS nor with the number of antihistamines necessary for symptom relief. In CU− we found a positive correlation of DAO levels with the frequency of oily and canned (also oily) fish intake. It has previously been shown that CU− and CU+ show different clinical and immunological features,13,24–26 such as the association of CU− with oily fish intake. This type of fish is able to generate more biogenic amines and could be an elicitor of symptoms in CU. Otherwise the anti-inflammatory properties of Ω-3 polyunsaturated fatty acids (PUFA) typical of oily fish have been associated with a more favourable prognosis of CU.13 We could speculate that in CU−, oily fish intake associated biogenic amines upregulate enteric DAO in order to deal with higher histamine amounts, which is finally reflected in higher serum DAO levels, more if CU− is associated with oily fish intake.13 However, this would not explain the lower levels of DAO in CU− versus CU+. Therefore, Ω-3 PUFA could be the components responsible for an overall lower serum DAO activity in CU−. In accordance with this idea, in an animal model of injured intestine, fish oil improved intestinal morphology and intestinal barrier function, decreasing plasma DAO activity and an increasing mucosal DAO activity.27 If lower serum DAO levels are associated with increased mucosal DAO, the mentioned study would also be in accordance with our findings, where in the subgroup of CU−, fish intake associated urticaria was not associated with different DAO levels, probably due to sufficient enteric DAO to deal with a biogenic amine rich meal.
On the other hand, in CU+, patients who claimed worsening of urticaria after fish intake had lower DAO levels. The main elicitors of FIAE were oily fish species (data not shown). This remembers food associated symptoms in histamine intolerance, where enteric extracellular DAO levels are too low in order to properly degrade biogenic amines, and is finally associated with generalised symptoms, including urticaria. In humans, the highest DAO activities are shown from small bowel and colon ascendens4 and therefore serum DAO reflects mainly production in the gut. Our serum samples do not reflect the exact DAO levels at the time of reaction, but if we take into account the relative stability of DAO over time, we can conclude that these lower levels predispose to urticaria worsening after fish intake. DAO forms the main barrier for intestinal histamine resorption,28 and adverse reactions to amine producing fish can be expected if dietary amines exceed the degrading capacity of enteric DAO.
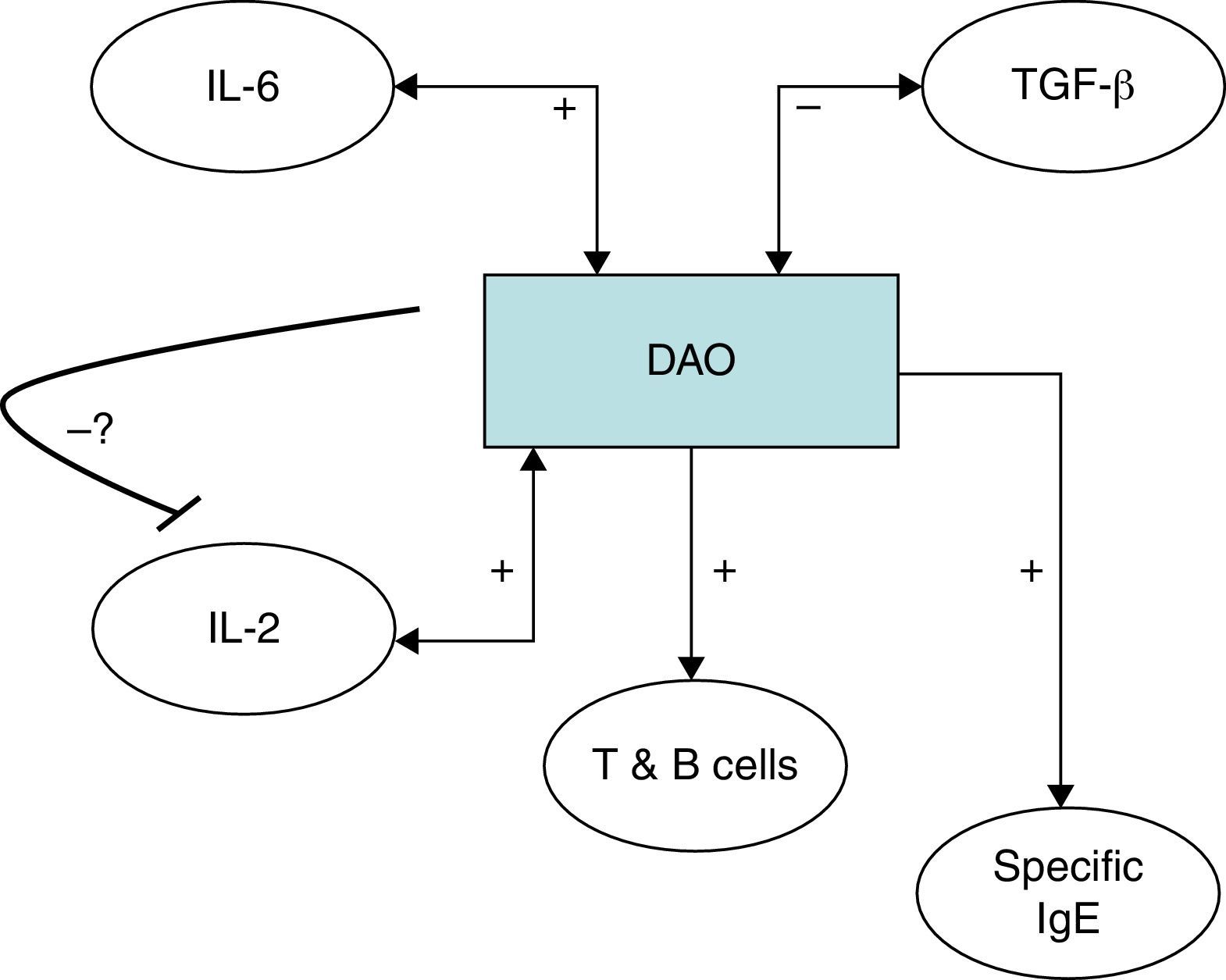
It has been proposed that DAO might be responsible for scavenging extracellular histamine after mediator release,29–31 a fact to consider in the setting of CU. It is therefore to be expected that DAO should be upregulated in CU in order to deal with higher amounts of mast-cell released histamine. In this scenario, we argue that the production of DAO is not sufficient for degrading surplus histamine and simultaneously scavenging food-borne histamine. Histamine intolerance results typically from a disequilibrium between production of enteric DAO and amine containing food. Likewise, post-heparin DAO activity in CU was only slightly reduced or close to the lower limit of the normal range in a previous study.3
Furthermore, it cannot be ruled out that in some cases of CU, intestinal barrier function is damaged, leading to elevated serum DAO.32 Polimeno et al. described an elevated intestinal permeability in Anisakis sensitised subjects.33 Likewise, Anisakis allergens have repeatedly been shown to provoke gastro-intestinal mucosal injury or motility disturbances in animal and human studies.34,35 Thus, our data are in concordance with these reports, where DAO serum elevation has been proposed to result from mucosal injury and elevated intestinal permeability.32 Maintz et al. reported a reduced histamine degradation capacity in a subgroup of patients with atopic eczema, where food allergy can coexist with an altered gastrointestinal mucosal barrier.29 Therefore, apparently normal DAO levels in CU could parallel an insufficient amount of enteric DAO for scavenging amine rich foods in the CU+ phenotype.
Gastrointestinal disturbances have previously been associated with urticaria in patients with reduced plasma DAO or histamine degradation capacity.36 We could not associate gastrointestinal symptoms after fish intake with lower DAO levels as could be hypothesised. This could be due to bias when assessing symptoms. Urticaria exacerbation is easy to assess by the patient and the physician, whereas gastrointestinal complaints and their relationship with fish intake are more inaccurate.
The second group of findings in our study was related to the immune profile associated with serum DAO. Anisakis-induced lymphocyte proliferation percentages were highly correlated with serum DAO. This is an expected event, as DAO is considered a regulating enzyme in rapidly proliferating tissues.9 Histamine and other polyamines are required in many cellular processes, especially for tissue proliferation. High polyamine levels are typical for actively proliferating cell populations and have been shown to be causative and not a result of cell growth.37 Here, DAO levels reflect the enzymatic role when regulating this response. In our setting, CU+ patients have higher DAO levels than CU− patients had, but in CU+ we also detected a positive correlation between DAO and specific IgE, serum IL-2 and IL-6. These relationships could be summarised in the following way: higher Anisakis IgE levels reflect a higher turnover of antibody producing cells, which is reflected by higher in vitro proliferation percentages, where IL-2 is known to be necessary for T cell proliferation.
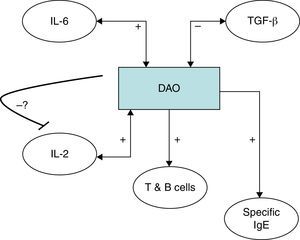
It has been shown that polyamine levels regulate IL-2 production by human peripheral blood mononuclear cells.38,39 It has however to be established if the relationship between the detected immune features and DAO can also be validated in subjects without urticaria, or otherwise is a specific situation in chronic urticaria and responsible for the chronic inflammatory state of this condition. IL-6 has previously been shown to be correlated with disease activity in CU26,40 and higher TGF-β has been detected in Anisakis sensitised versus non-sensitised CU patients.26 IL-6 as a pro-inflammatory cytokine fits in the detailed scenario with an elevated immune response; whereas the negative correlation of Con A induced TGF-β with DAO is antagonistic (Fig. 3). An alternative explanation would be that DAO is ultimately responsible for the rest of the encountered immune features, leading to a pro-inflammatory state. In this sense, frequent genetic polymorphisms associated with different DAO activities have been characterised41,42 which could be studied in the future for their relationship with the clinical and diet-associated features of CU. We cannot rule out that the potential effect of genetic variants as a putative factor may have influenced the findings. Thus, a DAO polymorphism in the DAO gene has been described to be associated with the clinical response in crossed-hypersensitivity to NSAIDs.42 Interestingly, a subgroup of patients with NSAID intolerance display cutaneous symptoms with urticaria. Further, gender-related variability of DAO has been reported previously,43 but our data showed no differences. Even if this could be due to the specific disease entity or to the fact that up to 80% of CU patients are female, we cannot rule out the influence of gender or genetic variants in our findings.
Serum diamine oxidase levels and their association with different immune markers in Anisakis sensitisation associated chronic urticaria (CU+): we detected a positive correlation between DAO and serum IL-2, Anisakis-induced lymphocyte proliferation and specific IgE production. Further DAO was also positively correlated with serum IL-6 but negatively with Concanavalin A induced TGF-β. The possible negative feedback-loop from DAO to IL-2 is shown because it has previously been shown that polyamine levels regulate IL-2 production by human peripheral blood mononuclear cells.37,38 It has to be established if the encountered relationships can be generalised or are CU+ specific.
When assessing serum DAO levels and the possible association with clinical features in CU, they should be interpreted taking into account the main factors acting on their levels: inter-individual variability, up-regulation by proliferating tissues, consumption by regulating features (histamine) and dietary factors. Finally, there are situations where an “excess” of dietary biogenic amines leads to clinically overt symptoms. It can therefore be expected that at least in a subgroup of CU, dietary measures addressing amine rich or histamine liberating food or available DAO substitution could be useful, as has been postulated previously.5,44
We did not have histamine concentrations available in serum in order to analyse its further interrelationship with DAO. This has been performed in a study of patients with CU comparing patients with and without gastro-intestinal symptoms.23 The authors did not find any association with clinical parameters but a clear and expectable negative correlation of histamine and DAO levels. Another study found decreased DAO activities but normal plasma histamine levels in patients with recurrent urticaria.2 In a third study, an amine-free diet had a clear effect lowering histamine levels, but DAO did not change.3
Taken together, our results show different serum-DAO levels depending on the CU phenotype. Especially in CU+, serum DAO was not only associated with different immune features, but was also clinically relevant when assessed as a marker of exacerbation on amine rich fish. Our results encourage future studies focusing on possible dietary advice and/or available DAO substitution in those CU patients with lower DAO levels and/or previous food induced exacerbation. Further, plant-derived DAO have also been proposed in the treatment of allergic disease.37
Ethical disclosuresPatients’ data protectionConfidentiality of data. The authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Protection of human subjects and animals in researchThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Conflict of interestThe authors declare no conflict of interest.
FundingThe study was funded by grants from Fundación Sociedad Española de Alergología e Inmunología Clínica (SEAIC, 2008/2009) and Fundación Mutua Madrileña (2009-20011), Spain.
The authors thank Duarte-Miguel de Sousa Teixeira for technical support.