INTRODUCTION
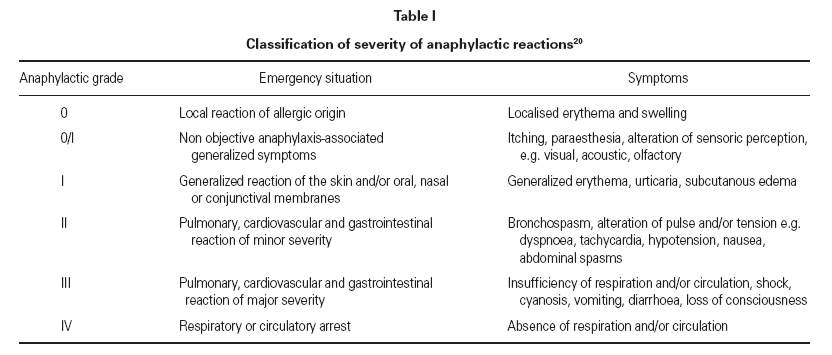
Specific immunotherapy using purified venom (venom immunotherapy) (VIT) is indicated in individuals who have experienced a hypersensitive systemic hymenoptera sting reaction 1-3 (table I).
A typical VIT schedule comprises two steps. The first is to increase doses reaching the maximum dose (usually 100 g of purified venom) over a number of days to several weeks. The second is to maintain the maximum dose and to extend the injection intervals (usually 4 to 6 weeks 1,4 ). A few groups have prolonged these intervals up to 12 weeks 5-8 . Termination of VIT is generally proposed after 3-5 years. There is no routine test available to prove the effect of VIT or a resensitization.
Relying on the observation that a wide variation with regard to the schedule provides safe protection we prolonged the intervals between maintenance VIT up to 6 months without temporary limitation. This design was to demonstrate safety, efficacy and tolerance to be comparable to that found with existing VIT protocols. In addition, this modified schedule was to achieve ongoing protection by continuation of maintenance therapy as well as to alleviate efforts and costs by widening the VIT intervals up to 6 months. This was conducted in a prospective study.
MATERIALS AND METHODS
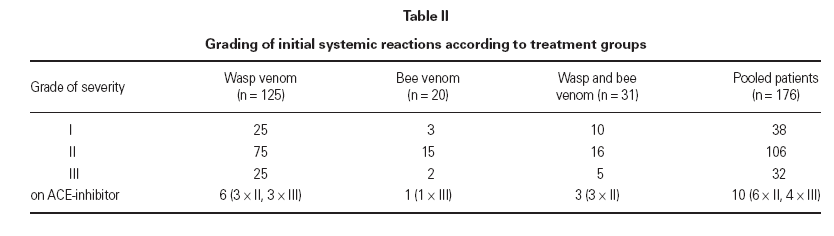
PatientsThe study involved a total of 176 patients (96 fe-males, 80 males; mean age, 52.5 years, age range 18-84 years). VIT indication criteria were a significant medical history of anaphylactic reaction in context with a positive skin test result and/or detection of venom specific IgE. All patients had experienced systemic reactions of different severities (table II). In cases of sensitization to both bee and wasp allergen we started a combined treatment schedule of wasp and bee venom. All patients gave their informed consent.
Table I Classification of severity of anaphylactic reactions 20
Table II Grading of initial systemic reactions according to treatment groups
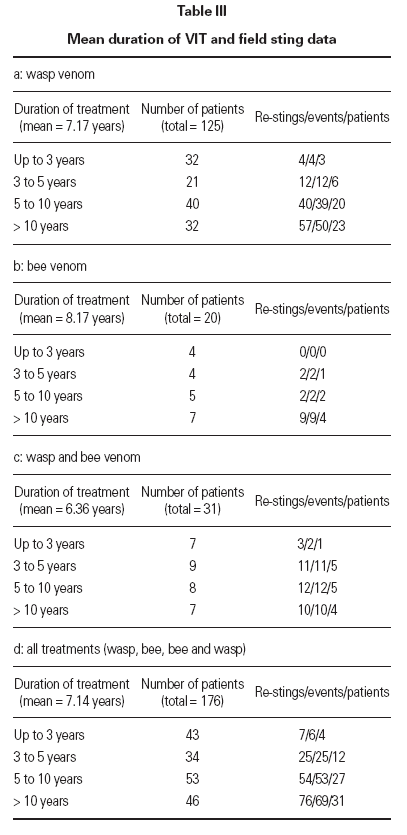
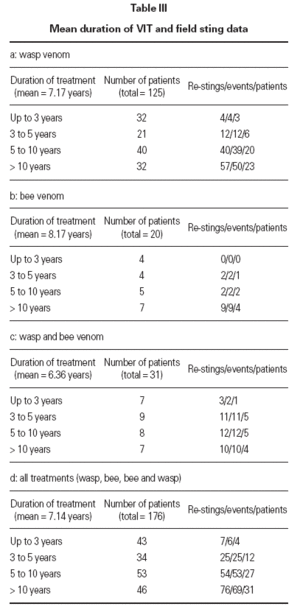
Honey bee venom (HBV) was given to 20 patients, wasp venom (WV) to 125 patients and both venoms to 31 patients. This comprised 176 treatments and 1256 treatment years. Six months maintenance phase in total is 1125 treatment years. The mean duration of treatment was 7.14 years (range 1.16-
25.49 years) as shown in table III.
Many patients dropped out of the study during the maintenance phase because they could not continue over several years. The majority moved away from our area or developed a non-related disease
(e.g. chemotherapy treatment) resulting in their ex-clusion. Controls to compare to conventional VIT schedules could not be recruited because of non-compliance over years following these schedules and the desire to be included in our special schedule.
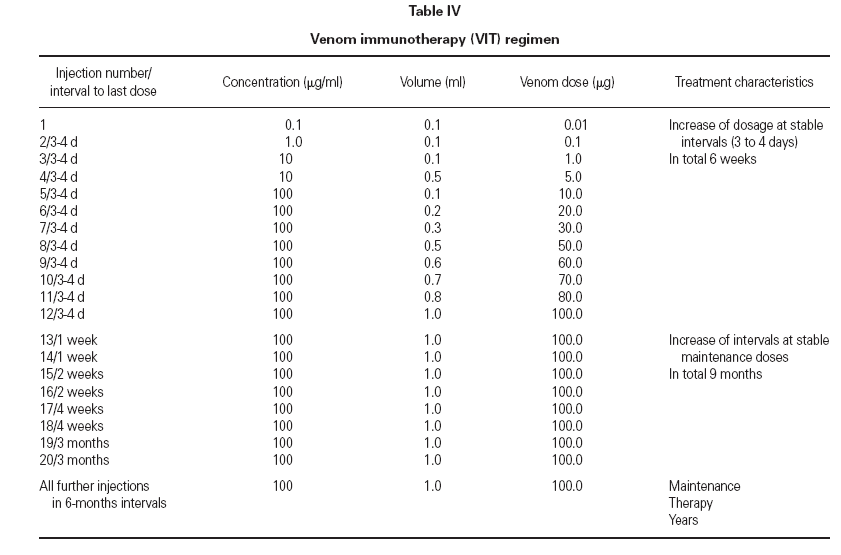
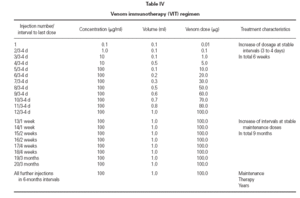
TreatmentTreatment was initiated in accordance with standard criteria. The protocol (table IV) provided an increasing dose from 0.01 g up to 100 g within a maximum time range of 6 weeks (typical injection intervals of 3 to 4 days). After reaching the maximum dose of 100 g the injection intervals were gradually extended up to 6 months, as shown in table IV, by maintaining the dose of 100 g venom per injection. Treatment was continued unless the patient wished to withdraw.
Consent was given after a statement of advantages and disadvantages concerning our treatment protocol compared to protocols applied in other centres. This was documented in the dossiers.
Aqueous solutions of HBV or WV were injected (Venomil ® , Bencard Allergy (ATL), Munich, Germany, or Reless ® , Scherax, Hamburg, Germany). Maintenance therapy was performed strictly every 6 months.
Table III Mean duration of VIT and field sting data
Table IV Venom immunotherapy (VIT) regimen
Deviations of several days were permitted for urgent reasons such as fever or holidays. Following an injection and a 30-minute observation period of the patient, the local reaction was measured and documented in the ward. In response to any complaint the observation was extended. In a few cases pharmacological intervention was necessary to counteract systemic side effects of venom injection.
Documentation of tolerance to therapy and sting reactionsEvery venom injection was preceeded by a medical interview for current medication, new complaints, acute infectious or allergic signs, tolerance of last VIT injection, occurrence and course of any new hymenoptera stings. Reactions resulting from VIT or field stings were documented in the patient’s report.
Serological evaluationSequential measurements of serum IgE (total IgE) and venom specific IgE were made before starting VIT and repeated regularly during long term treatment. For determination of venom specific antibodies over the last 25 years we specifically used standardized assay procedures (RAST, CAP) available from Pharmacia (Uppsala, Sweden). Results were expressed in U/ml and according to the manu-facturer’s scoring system and documented in the patient’s dossier. From the start of our study only RAST scores (and not discrete units of specific IgE) were available. Thus for these cases we created a mean value from the RAST class as a surrogate parameter.
RESULTS
Tolerance and safetyGenerally, VIT was tolerated very well. All of the patients in this study had reached the stage of maintenance treatment in the 6 months treatment interval. Patients experienced local reactions such as itching and swelling around injection site, which disappeared spontaneously within 24 hours post-in-jection. Patients who complained were allowed to take antihistamines at the evening before treatment day. Local reactions never exceeded 10 cm in diameter. The risk of development of systemic reactions to venom injection, which occurred rarely in this study, did not correspond with the size of local reactions.
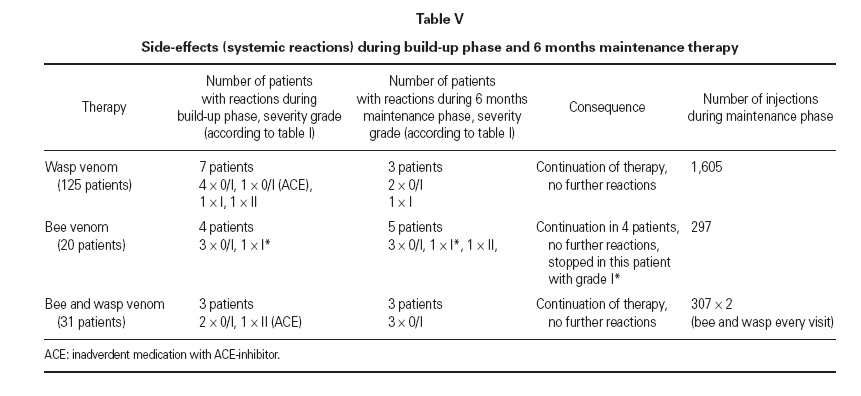
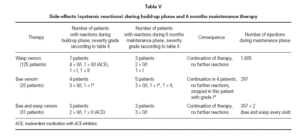
Table V Side-effects (systemic reactions) during build-up phase and 6 months maintenance therapy
Systemic reactions to a single or a few injections were observed in 11 patients (6.3 %) (3 treated with wasp venom, 5 treated with bee venom, 3 with combined treatment) during maintenance therapy, whereas during the build-up phase a total of 14 patients (8.0 %) had systemic reactions. These side effects mostly appeared within 15 minutes following subcutaneous injections and were usually mild, i.e. maximum gradeI (table V). In one case (bee venom) after 6 years of maintenance injections with the maximum dose without any systemic side effect, an anaphylactic reaction of grade II developed after injection. This reaction did not recur after further injections that were given without change of protocol. Another patient that showed a grade II reaction during the build up phase was without reactions in all subsequent Injections that included even higher dosages. Because of an inadvertent comedication with ACE-inhibitor for hypertension that was taken in spite of better knowledge, two patients reacted during the build-up phase (1 × grade 0/1, 1 × grade II). After discontinuation of this medication treatment was well tolerated. In only one patient treatment was stopped because of ongoing grade I reactions to the maintenance dose (bee ven-om). In this patient already during build-up phase the maximum dose provoked the same extent of reaction.
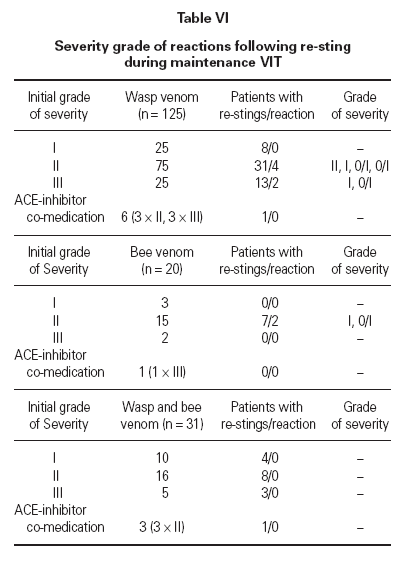
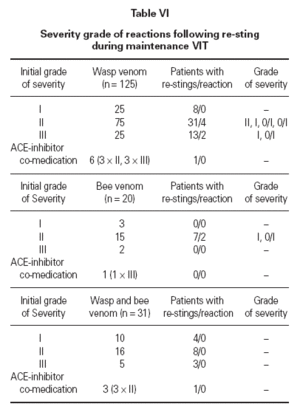
Determination of efficacy by re-sting reactionsIn 74 patients (42 %) under maintenance therapy, a total of 153 hymenoptera attacks occurred (events), comprising a total of 162 field stings. For wasp ven-om VIT, there were 113 wasp stings in 105 events in 52 patients. For bee venom VIT, there were 13 bee stings in 13 events in 7 patients. For combined VIT, there were 36 hymenoptera stings in 35 events in 15 patients. Systemic reactions re-occurred in 8 events (8 stings, 8 patients) during 6 months maintenance therapy (10.8 % of patients stung, 4.5 % of all patients). In 7 out of the 8 patients with persistent anaphylactic reactivity (in spite of therapy) re-sting reaction was of lower intensity compared to the initial reaction (1 × grade III → grade I, 1 × grade III → grade 0/1, 2 × grade II → grade I, 3 × grade II → grade 0/I) whereas in one patient re-sting reaction remained a grade II reaction (table VI). This patient was judged as a therapy failure, whereas in all other re-sting reactions the initial life-threatening situation was eliminated. Thus patients were stable after the field sting and pharmacological intervention was not regarded as necessary.
Efficacy of venom treatment with 6 months maintenance intervals was 98.6 % (73 of 74 patients restung showed minor or no reactions) or 99.4 % regarding the number of total stings received during maintenance therapy. There was no correlation between duration of the maintenance therapy at the time of the re-sting and the severity of the reaction. Neither could we find a connection between the reaction severity after the re-sting and the reaction severity after the initial sting (table VI).
Table VI Severity grade of reactions following re-sting during maintenance VIT
Serological results
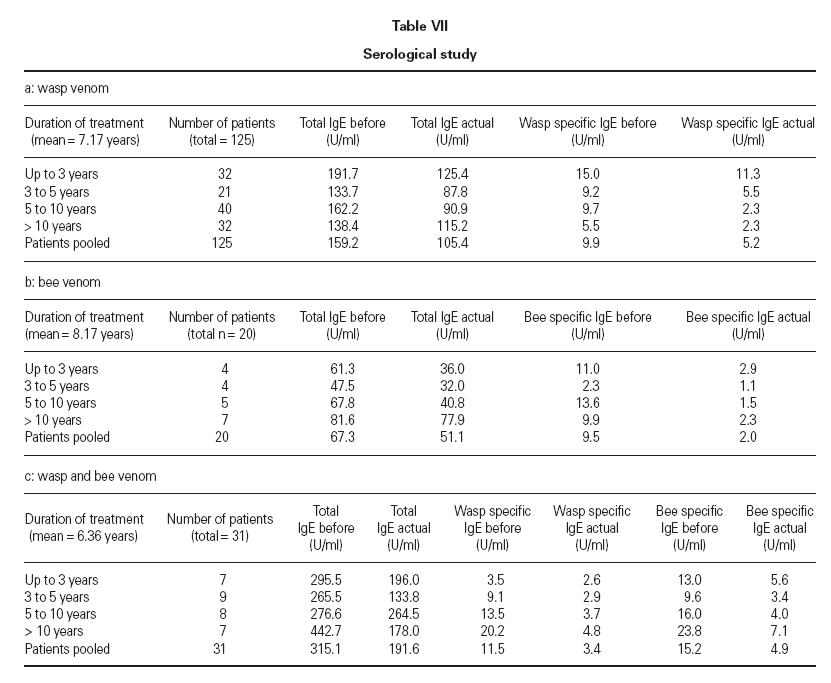
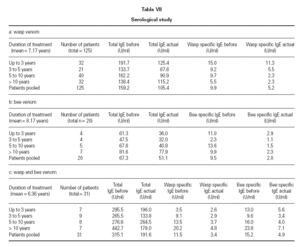
Before and during treatment total IgE and aller-gen-specific IgE was monitored. Total IgE and specific IgE was expressed in U/ml. Additionally the RAST and CAP scoring system was used, according to the laboratory test provider. The mean value (arithmetic mean) of total IgE from 125 patients on wasp venom therapy decreased from 159.2 U/ml before start of therapy to a mean value of 105.4 U/ml. This was paralleled by a decrease of the arithmetic mean of wasp specific IgE, i.e. 9.9 U/ml before treatment and 5.2 U/ml after treatment. This decrease was not observed when comparing the median of the manu-facturer’s scoring system (RAST/CAP class 2 before and after treatment). The corresponding values in the bee venom group and the combined treatment group are listed in table VII.
DISCUSSIONFor over 25 years VIT has been demonstrated as an efficacious treatment for hypersensitivity to hymenoptera venom 9-11 . However, the criteria for the maintenance therapy protocol still remain debatable. In particular, the optimal duration is still not known but is suggested between 3 and 5 years according to different authors 1,4,12,13 and based on results from sting challenge 14-18 .
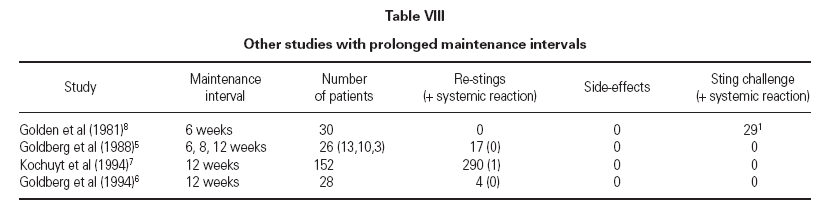
Our therapeutic modification extended VIT maintenance dose intervals gradually to 6 months. A control group was not recruitable over the same time period. Incidence and severity of side-effects in our group did not differ from those observed in other studies with shorter intervals (table VIII) 9,10 .
Side-effects were calculated as a percentage, comparing the number of patients with reactions to the total number of treated patients. The number of patients with mild systemic reactions was lower during the 6 months maintenance phase (6.3 %) than during the initial build-up phase (8.0 %). However, comparability is not exact, because of the different settings in both treatment phases and also because there have been more injections in the build-up phase than in the maintenance phase (20 versus 13 injections calculated on the average duration of treatment). The frequency of systemic side-effects compares well to the results obtained from conventional protocols using standard maintenance intervals. As reported in other studies 10 , most of subjects showing systemic side effects suffered from allergy to bee venom or had a combination treatment. One female patient treated with bee venom finally stopped treatment because of ongoing grade I reactions, during both the build-up phase and the six months maintenance phase.
The efficacy of the presented regimen was assessed by the effects of 162 re-stings with the culprit insect attacking 74 patients during maintenance therapy (details in table III). Mild systemic reactions (non objective anaphylactic symptoms: grade 0/I) were developed in 4 patients. In 3 patients re-sting reactions were grade I and thus significantly less pronounced than before commencing VIT (2 of them experienced a grade II and 1 patient a grade III initial sting reaction, see table VI). In only one patient the initial and re-sting reactions remained unchanged. In total, 4/74 (5.4 %) patients expressed objective anaphylactic symptoms (3x generalized skin reaction, 1x cardiovascular reaction of grade II) in the re-sting situation and 4/74 (5.4 %) patients showed non-objective systemic reactions after re-sting. Other groups have reported systemic reactions after re-sting in 3-23 % of patients receiving one dose a month 19 .
We paid particular attention to patients taking ACE-inhibitors which may enhance anaphylactic reactions. In 10 patients an anaphylactic sting reaction occurred whilst under medication with an ACE-inhibitor. After discontinuing the medication, VIT did not result in any pronounced side-effects. Two re-stings were reported in this small group without a following reaction.
Analysis of specific IgE from serum samples showed a mean decrease of about 50 %. As specific IgE only rarely disappears following VIT these results are congruent to IgE monitoring in other insect allergen treatment schedules2,9,10. There was no substantial correlation to the side-effects or the reactions following re-sting. The same holds true for the skin-test data (not shown), rendering a reduction in the mean diameter without observation of negative tests. Thus, the measurement of specific IgE and skin-testing in our study was appropriate for diagnosis but not for monitoring.
Table VII Serological study
Table VIII Other studies with prolonged maintenance intervals
In conclusion, this study has demonstrated the efficacy and safety of long-term VIT incorporating 6-month maintenance therapy intervals. Results are comparable to that found following the standard protocols used by other groups, particularly with regard to success following re-sting. Regarding side-effects, local reactions during six months intervals did not differ from local reactions in the short intervals after reaching the maximum dose (100 g). The occurrence of systemic reactions to therapy was not higher than in the protocols reported in the literature and have been easily controlled by an adapted antiallergic treatment 20 . No patient had to be hospitalized for a systemic reaction to VIT.
It is established that long-term VIT with continuous maintenance therapy provides long-term protection. This study has provided an useful alternative schedule which is economical in time for both doctor and patient. In addition, the patients benefit from continuous monitoring and hence a superior life quality due to the resulting long-time protection.
Efficacy of long term treatment is finally illustrated by a case of wasp venom allergy. The patient who initially showed a wasp sting reaction of grade II had two re-stings during a treatment period of four years without anaphylactic symptoms. Six years after unilateral cessation of treatment another re-sting provoked a de-novo grade II reaction and hence therapy was re-started.
Finally, additional aspects and data have to be mentioned. During the last 25 years more than 1000 patients received the build-up phase of VIT in our clinic. Maintenance therapy according to our protocol was given by their family doctors and no serious or severe side effects were reported. In some patients therapy was interrupted for various reasons, e.g. for pregnancy, but could be resumed using our protocol without dose reduction. The findings indicate that our long-term 6-month protocol provides sufficient security, even for prolongation of the maintenance interval. As to our experience the maintenance protocol can be subsequently added to any previous protocol bringing up the dose to 100 g. Of particular interest is the finding in our study that also older patients tolerated this modification of VIT and benefited by it.
Correspondence:
H.W. BaenklerDepartment of Allergy Medical University Hospital III Krankenhausstraße, 12 91054 Erlangen, Germany