INTRODUCTION
The spectrum of cutaneous eruptions in association with calcium channel blockers is extensive, varying from exanthemas to severe adverse events (erythema multiforme, Stevens-Johnson syndrome, toxic epidermal necrolysis, hypersensitivity syndrome reaction, acute generalized exanthematous pustulosis...) 1-12. Reactions due to diltiazem occur more frequently than with other calcium channel blockers 1-4. The mechanism of these reactions is unknown, and it is likely that the pathophysiology differs with each reaction. Patch testing has been used as confirmatory testing in patients with extensive cutaneous reactions 2,4,5,7-11. Cross-reactivity among these drugs have not been established.
CASE REPORTS
Case 1
A 54-year-old man developed a generalized eythema-multiforme-like reaction followed by erythrodermia and exfoliative dermatitis 6-7 days after starting on diltiazem. He had increased white blood cell count with eosinophilia. A skin biopsy showed intraepidermal vesicular dermatitis, ballooning degeneration of keratinocytes and mixed-cell infiltrate. The drug was stopped and remission was obtained with emollients and systemic corticosteroids and antihistamines within 12 days.
Case 2
A 80-year-old woman experienced a pruritic exanthematous eruption on her trunk which evolved to generalized erythrodermia and superficial desquamation. This reaction appeared 10 days after taking diltiazem, and gradually improved in 10-12 days after discontinuation of this drug. We have no information about the treatment administered to the patient.
Case 3
A 79-year-old man began treatment with diltiazem for managing hypertension. Three days later, he presented with erythema and pruritus initially on the back, and then affecting thorax, extremities and face. Diltiazem was stopped and steroid and antihistamine therapy was given. His skin condition improved, but 3 days later the patient received verapamil with worsening of previous situation. He recovered within 7 days.
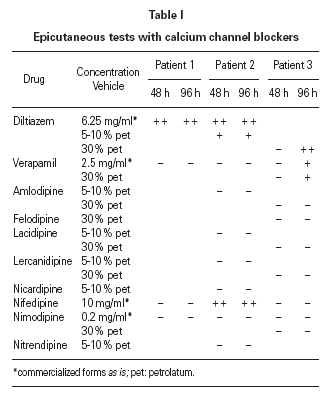
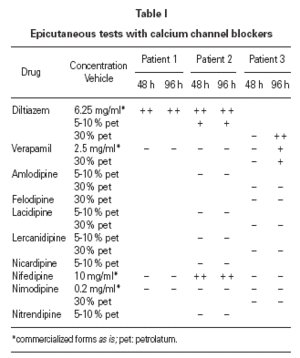
Two to six months after the reactions, we carried out epicutaneous tests with calcium channel blockers from different groups: benzothiazepines (diltiazem), phenylalkylamines (verapamil), and dihydropyridines (nifedipine, nimodipine, nicardipine, nitrendipine, amlodipine, lacidipine and lercanidipine). Diltiazem proved positive (at 48 and 96 hours) in the three patients; nifedipine was also positive in patient 2, and verapamil in patient 3 (table I). The same tests were negative in 10 control patients. Controlled administration of verapamil was well tolerated in patient 2 after the reaction, and the patient 1 has taken nifedipine without problems.
DISCUSSION
Maculopapular exanthema is the most common cutaneous adverse reaction to diltiazem. We describe 3 patients with different cutaneous reactions due to this drug (eythema-multiforme-like reaction, erythrodermia and a pruritic exanthematous eruption).
According to the literature, patch tests with diltiazem are useful in diagnosing cutaneous adverse reactions with this drug. Positive results have been reported using commercialized forms of diltiazem as is 5-6 or diluted at 10 mg/ml 8, 1 % pet 7,10,11, 5-10 % in pet or water 9 or 30 % in water, petrolatum or alcohol 4. In case of severe reactions, these tests should be performed with caution and beginning with low concentrations 4,13.
There are no consensus about cross reactions among calcium channel blockers. The review of the literature shows few papers referred to this subject and different results 2,4-6,10,11. It is difficult to come to conclusions as the clinical presentation of patients and methodology used for the diagnosis are different. Some authors suggest cross reactions between diltiazem and nifedipine 12, amlodipine 6 or verapamil 4 (by patch tests 2,4 or regarding clinical reactions 6,12), but no others 5,10,11. In our case, all the patients had positive patch tests with diltiazem. In addition to that, the patient 2 had positive patch tests with nifedipine (but tolerated verapamil), and the patient 3 with verapamil (in fact, he worsened when he took this drug). It is difficult to establish the meaning of this results as we have not performed controlled administration of these drugs.
Regarding our results and others previously reported, in case of adverse cutaneous reactions to diltiazem, we suggest to perform patch tests with this drug and other calcium channel blockers (the concentrations should be established taking into account the severity of the reaction) 13. Once confirmed the positivity of diltiazem, it would be possible to administer some of the calcium channel blockers with negative patch test result under hospital surveillance.
Conclusions: 1) We report 3 cases of cutaneous reactions due to diltiazem. 2) Epicutaneous tests have been useful for diagnosis. 3) Although one of our patients had positive patch tests to diltiazem and nifedipine and the other one with diltiazem and verapamil, more studies are needed to establish cross reactions among calcium channel blockers.