From the paediatric point of view, we have undertaken two Delphi studies into bronchial asthma. The first is related to the consensus known as the consensus document of the five associations. The second is more recent and has been undertaken with GEMA (the Spanish Guidelines on the Management of Asthma).
The aim of this paper is to carry out a descriptive study comparing the 2 Delphi processes and to objectively assess if in some way behaviour over the past two years has changed as far as expert opinion is concerned.
In the consensus document those points giving rise to most controversy were the treatment of children under three years of age and treatment with immunotherapy in allergic asthma. It is also necessary to highlight how important it was at that particular point in time to define the phenotypes of wheezing and the predictive index of asthma in children of less than 3 years of age. Of the 52 questions in the questionnaire, in 13.6% the panel of experts reached no consensus in their positions.
Following GEMA the Delphi methodology, 56 questions were asked in the first round of the questionnaire, and consensus was reached in 87.5%. As regards the paediatric part relating to diagnosis and treatment in children, agreement was reached on all the questions in the first round. Agreement was reached in 8.92% questions in the second round.
Clinical guidelines and consensus documents can modify behaviour towards an illness, both in the diagnosis and treatment.
The Delphi method, which takes its inspiration from the ancient oracle of Delphos, was developed as an instrument to make predictions in the case of a nuclear catastrophe.1 Listone et al.2 define the Delphi technique as a way of structuring a process of group communication which is effective in that it allows a group of individuals, as a whole, to deal with a complex process such as asthma.
From the paediatric point of view, we have undertaken two Delphi studies into bronchial asthma. The first is related to consensus and is known as the consensus document of the five associations.3,4 The second is more recent and has been undertaken with GEMA (Guía Española del Manejo del Asma (the Spanish Guidelines on the Management of Asthma)) covering all the aspects of asthma relevant to paediatrics.
The aim of this paper is to carry out a descriptive study comparing the two Delphi processes and to objectively assess if in some way behaviour over the past two years has changed as far as expert opinion is concerned.
The Delphi Method in the Two Consensus DocumentsIn both studies a group of experts were selected who were then asked their opinion on different issues from the consensus document and from the guidelines. This reinforces and ensures a greater quality of the work undertaken, since most of the experts in asthma in Spain were included.
Using successive questionnaires, the objective is to reduce the interquartile range, or rather, how much the opinion of one expert deviates from the opinion of the group, thus allowing the median to be calculated. In the first questionnaire the interquartile range is calculated and in the second questionnaire, with knowledge of the opinions of the other experts, a discussion is begun with the aim of reaching a consensus on the topic.
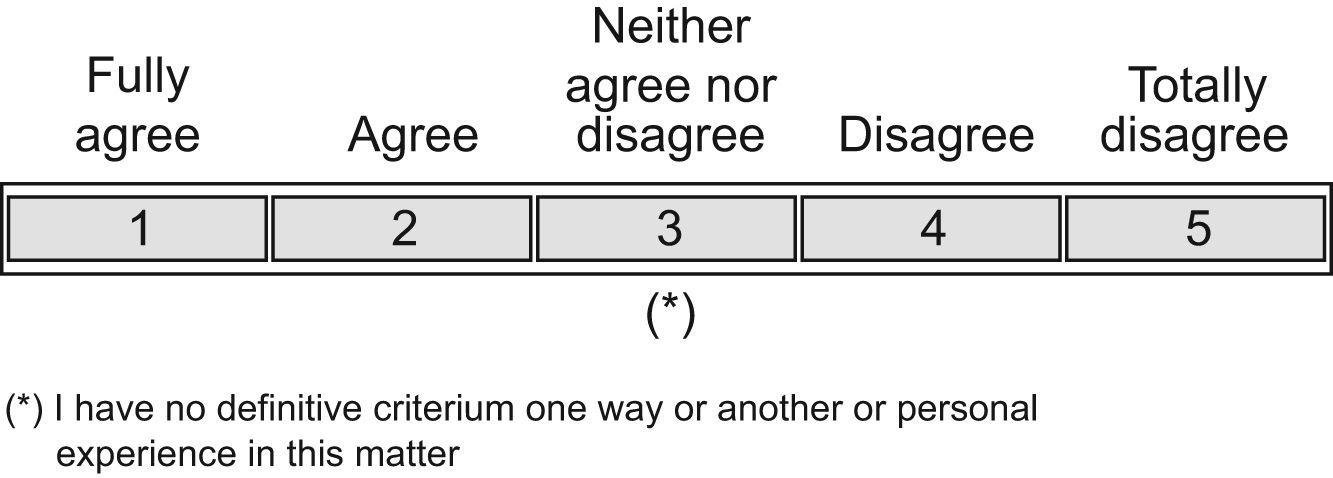
The questions must be precise, quantifiable and independent. The questions had to be answered in the following way: totally agree, agree, neither agree nor disagree, disagree, or totally disagree (Figure 1).
The interpretation of the results consists in the analysis of the average group score for each item and in establishing the 95% confidence interval (95% CI). The higher the average (the nearer it is to 5), the greater the disagreement, and the lower the average (the nearer it is to 1) the greater the agreement between the experts on the question posed. If the 95% CI includes the score of 3 (neither agree nor disagree) the group has reached no consensus neither positive nor negative and so neither accepts nor rejects the item in question (Figure 2).
In those items in which no consensus was reached after completion of the two questionnaires, the distribution of the replies was analysed to check whether markedly different opinions existed between the experts or whether the majority of the group had insufficient criteria.
Consensus Document on the Management of Childhood AsthmaThe consensus document drawn up by the five paediatric associations (the Spanish Society of Paediatric Allergy and Clinical Immunology; the Spanish Society of Paediatric Pneumology; the Spanish Society of Outpatient and Primary Care Paediatrics; The Spanish Association of Paediatrics and Primary Care; and the Spanish Society of Emergency Paediatrics) was first published in the journal Anales Españoles de Pediatría and in Allergologia et Immunopathologia.3,4 In spite of representing a group of different specialists dealing with asthma and some initial points of disagreement, following a series of literature reviews and the application of criteria of scientific evidence, a consensus was reached in the final version of the document. Those points giving rise to most controversy were the treatment of children under 3 years of age and treatment with immunotherapy in allergic asthma. It is also necessary to highlight how important it was at that particular point in time to define the phenotypes of wheezing and the predictive index of asthma in children under 3 years of age.
Subsequently the document underwent a Delphi process with child asthma experts from the different participating associations. The text of the consensus document was divided into 52 questions which were then copied into two questionnaires. The summary of this Delphi process, as can be seen in Figure 3, achieved a consensus in the first round on 42 questions (80.7%) in the questionnaire. Agreement on three questions (5.7%) was reached in the second round of the questionnaire (two on immunotherapy and one related to treatment with antileukotrienes). Of the 52 questions in the questionnaire, on seven (13.6%) the panel of experts reached no consensus in their positions (three relating to symptomatic treatment, three relating to immunotherapy and one question on who should assume the responsibility of educating the asthmatic patient).
GEMA 2009The 2009 Spanish Guidelines on the Management of Asthma (GEMA)5 is a consensus document for the diagnosis and treatment of asthma. Nine scientific associations participated, along with the Patient Forum, and methodological help was received from the South American Cochrane Center. In the paediatric aspect, the Spanish Society of Paediatric Pneumology and the Spanish Society of Paediatric Allergy and Clinical Immunology both participated. What makes these guidelines particularly interesting is that, unlike in other guidelines, childhood asthma has been included not as a special situation within asthma but is dealt with throughout the guidelines and within its different sections on diagnosis and treatment, which is what fundamentally distinguishes this disease in children.
As occurred in the Spanish Consensus Document on the management of childhood asthma, a Delphi study was carried out which involved 74 experts from different specialties. In the paediatric specialist part, eight were paediatric pneumologists and nine were paediatric allergists. However, the questionnaire was distributed to all the specialists involved. When the Delphi process was analysed by specialties in the two items in which consensus was not achieved, no significant differences were found between the replies of the different specialists. This may also be due to the size of the sample.
Following the Delphi methodology, 56 questions were asked and in the first round of the questionnaire, consensus was reached in 49 (87.5%). As regards the paediatric part relating to diagnosis and treatment in children, agreement was reached on all the questions in the first round. Agreement was reached in five (8.92%) questions in the second round, three of which related to the diagnosis and treatment of the disease and two to symptomatic treatment and indications for immunotherapy.
Rhinitis was also included in GEMA. In this aspect, no consensus was reached in two questions relating to diagnosis until the second round.
In only two (3.6%) of the 56 questions no consensus was reached and only one of these involved paediatric populations and was related to the control of environmental measures.
DiscussionIn view of the above, it is clear that the work of different specialists attempting to reach a consensus has repercussions on the management of the disease by paediatricians and as a result, patients also benefit.
If we first analyse similar questionsThe Consensus Document on the Management of Childhood Asthma stated: Specific immunotherapy is indicated if the following criteria are met: frequent episodic or persistent moderate asthma, mediated by IgE, when there is sensitisation to one single allergen or one predominant allergen or to a group of allergens with cross-reactivity. Drug treatment (and avoidance of the allergen) does not control the symptoms or causes adverse effects or the patient (or his or her parents or legal guardians) is unwilling to continue long-term treatment. The patient exhibits both nasal and lung symptoms.
The average score was 2.35 with a 95% CI of 1.7–3.0 and a median of 2. No consensus was reached between the panel of experts even though the text was included in the consensus document (Figure 4).
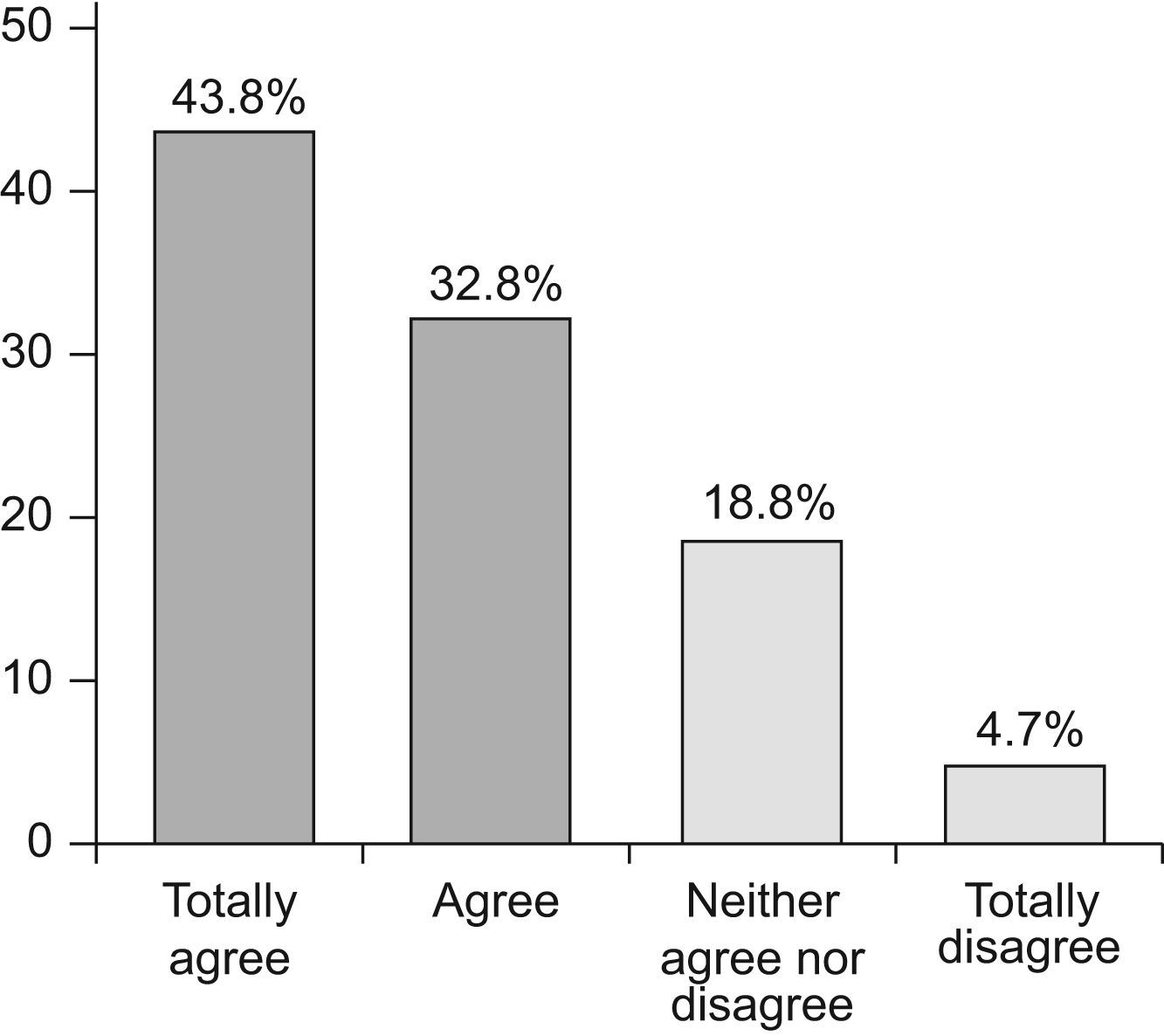
In GEMA it was also stated that: In the treatment of children with allergic asthma the use of immunotherapy should be considered whenever biologically standardised extracts are used and the patients are appropriately selected. The expert panel reached a majority consensus agreeing with the statement, with an average agreement score of 1.89 (95% CI 1.63–2.15) and a median of 2 (Figure 5).
On two questions in the childhood asthma consensus document no agreement was reached. These related to the use of sublingual immunotherapy and the age at which to begin immunotherapy. As no such questions were posed in GEMA, it is not possible to offer any comparisons.
For child allergists, the inclusion of immunotherapy in the childhood asthma consensus document constitutes an achievement even though no agreement was reached through the Delphi process. After two years, we consider it a further achievement that a majority consensus has been reached between different specialists in which treatment with immunotherapy in childhood allergic asthma is accepted, provided that the patients are well selected and the appropriate extracts are used. It is clear that a consensus is reached by sometimes accepting (as specialists) statements with which one may not always agree but which are not negated by scientific evidence.
In the case of the childhood asthma consensus document, the Delphi process revealed that 26.1% of experts disagree with the statement made in the question whilst the GEMA Delphi process showed only 4.7% of experts to be in disagreement. These data mean that immunotherapy in selected patients, with standardised extracts, as described in both the childhood asthma consensus document and GEMA, must be considered in the treatment of allergic asthma. Furthermore, it underlines the importance of guidelines and consensus documents for unifying criteria supported by scientific evidence and strength of recommendation.
If we analyse questions in which agreement was reached in the second round of the questionnaire in the childhood asthma consensus document and compare this with GEMA, we can see treatment strategy has been changed.
In the childhood asthma consensus document it was statedLeukotriene receptor antagonists may be prescribed as an alternative to inhaled corticoids (or in combination with them) to reduce bronchial inflammation in atopic children. The average group score was 2.13 (95% CI 1.64–2.62) and a median of 2. Disagreement reached 17.3% (Figure 6).
In the 2009 GEMA it was statedAs an alternative (in persistent moderate asthma) treatment may be begun with a combination on low-dose inhaled glucocorticoids plus an antileukotriene in children under 4 or a long-acting beta-2 adrenergic agonist in children over the age of 4. Consensus was reached in the first round of the questionnaire with an average group score of 1.52 (95% CI 1.34–1.69) and a median of 1. 1.5% of the panellist did not agree (Figure 7).
If we bear in mind that in the childhood asthma consensus document treatment of children over the age of 4 with long acting beta 2 adrenoceptor agonists always associated with inhaled glucocorticoids was accepted in the first round with a median score of 1.5, we interpret these results to mean that antileukotrienes have assumed their rightful position and the combined therapy is currently accepted to reduce the dose of corticoid or achieve better control with its associated use.
In conclusion, clinical guidelines and consensus documents based on scientific evidence and accompanied by strength of recommendation can modify behaviour towards an illness in which, both in the diagnosis and treatment, different specialists are involved, thus achieving a common way of acting. Furthermore, the ability to reach agreement implies an attitude of change and openness to new diagnostic techniques and new treatments, and from this those who will ultimately most benefit will be the patients themselves.
Conflict of interestNone to disclose.
We wish to thank Luzan 5 for providing the data on the Delphi Study.