INTRODUCTION
Asthmatic patients frequently report cold and upper respiratory symptoms some days before the asthma attack. The evidences correlating this infection and acute attacks have been reported since 1957 after the report that an epidemy of influenza A had triggered bronchospasm in two large American populations4. Several studies performed in children of different ages who presented wheezing, have isolated respiratory virus during acute attacks of bronchospasm. Viral identification was reported as being from 14 to 49 %2-13.
Other aspect to be considered is that asthmatic children usually present more severe symptomatology of lower respiratory tract after viral respiratory infections, because there is an additional effect of viral infection to preexisting pulmonary modifications in asthmatics12-16. Despite the studies developed in children sustain the hypothesis of viral respiratory infections triggering acute attacks of asthma, the results in adults are controversial12,17,18.
These associations previously described correlating respiratory infections, viral identification and acute asthma attacks suggest that there is a causal relationship specially in children10,12. Viral identification in asthmatics coincide with the increased use of medication, worsening of clinical symptoms and reduced daily FEV12,3,8,19,20. According to Hygiene Hypothesis, certain infections may actually prevent the development of allergic respiratory tract diseases, including asthma21.
There is no previous report developed in our country identifying viral agents during asthma attacks. Then, considering a group of asthmatic and non asthmatic children during viral infection of upper respiratory tract (URTI) or without it, the aim of this study was to detect the presence of adenovirus, respiratory syncytial virus, parainfluenza 1,2 and 3 and influenza A and B; and to correlate the viral isolation with clinical manifestations in older children.
PATIENTS AND METHODS
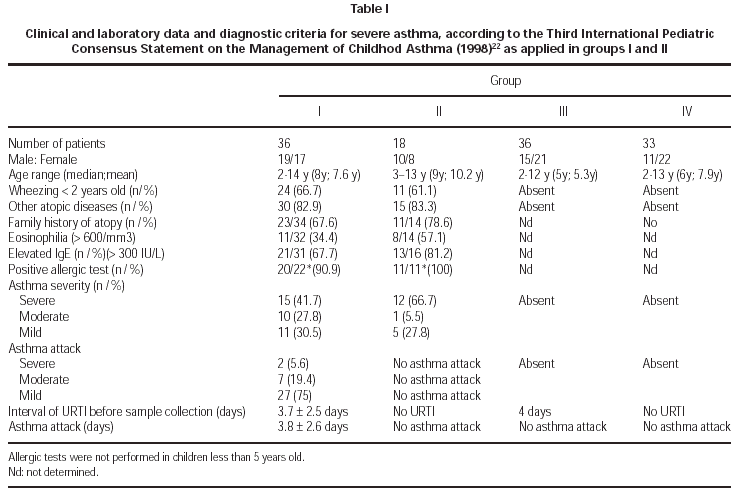
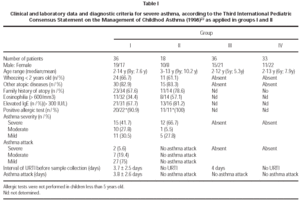
A hundred and five children (2-14 years old; mean 7,3 years) were included and 123 samples from nasal mucosa were collected. Most of the samples were collected during the winter time (105/123; 84.4 %) and 22/36 from GI and 13/18 patients from GII referred asthma attacks during the winter. The diagnosis and severity of asthma were established according to the IIIrd. International Pediatric Consensus Statement on the Management of Childhood Asthma, based on symptomatology, evaluation of pulmonary function and exclusion of other diseases22. All the patients were evaluated by the same physician and they were divided in the following groups: GI asthmatics with symptoms of URTI and acute asthma attack (n = 36); GII asthmatics from GI without symptoms of URTI, evaluated 3-8 weeks later GII (n = 18); GIII non asthmatic and non atopic children with URTI GIII (n = 36); GIV healthy children without URTI (n = 33). Two GI samples (35 and 36) belong to the same patient, both were collected in the presence of URTI, with an interval of 35 days in between. The period of collection between groups I and II was 20-48 days (mean 30.9 days). These data were considered separatelly for GI and GII, in order to apply statistical analysis, although they represent the same children in two phases. The patients were submitted to the following complementary tests: eosinophil count (eosinophilia number > 600 cells/mm3), dosage of serum IgE levels (> 300 IU/mL), thoracic X ray, pulmonary functional tests (older than 7 years old) and prick tests for inhalants (older than 5 years old) including Dermatophagoides pteronyssinus, Blomia tropicalis, cat and dog dander, house dust, a mixture of fungal antigens and wool. Positivity of prick tests was considered for wheal's size above positive control (histamine).
Acute asthma attack was identified by the presence of prolonged expiration, wheezing, tachypnea and/or dyspnea and/or intercostal retraction; these symptoms were used to classify the severity of the attack. Upper respiratory tract infection was considered between one and 7 days of symptomatology, in the presence of 3 or more complaints in asthmatics and, at least, 2 or more symptoms in non asthmatics, as follows: cough and/or sneezing, acute rhinorrhea, nasal obstruction, hypertrophy of turbinates, pain and/or hyperemia in retropharynx, headache and fever23.
Viral identification was performed in samples from nasal mucosa, collected with sterile swab and the material was kept in PBS 0.01M pH 7.2. The material was sent on ice to the laboratory. Antibiotics such as penicillin and streptomicin 1 % were used to control bacterial contamination.
Cell cultures and detection of viral antigens by indirect immunofluorescence: Viral identification was performed in cell cultures, using two cell lines: HEp-2 (laringeal carcinoma) and HeLa-I (uterus carcinoma), kept on MEM (essential human medium) with 10 % of bovine fetal serum, penicillin and streptomicin, replicated with ATV solution (0.2 % trypsin 1/250 Sigma and 0.02 % EDTA), according to previous protocol described by GARDNER, McQUILLIN, (1968)24. The cells were cultivated in microplates of 24 wells (Cornning, USA), up to 70-80 % of confluence. After the samples'innoculation, the plates were centrifuged and incubated in a water-jacketed incubator at 37 °C with atmosphere of 5 % CO2. The cytopathic effect during 15 days, read in inverted optical microscope. If positive, two additional cultures were performed and the virus was identified by indirect immunofluorescence. If negative, it was followed by two passages in cell cultures in order to confirm the negative result.
Indirect immunofluorescence: The samples were centrifuged and washed twice with PBS 0.01M pH 7.2. After the last washing, the sediment was suspended in 250 to 300 μl of PBS in order to obtain about 20 cells/field, with 200X objective. Two slides were prepared: one for screening, which 20 μl of each sample added in two holes and the other, with specific monoclonal antibodies where 20 μl of each sample were dropped in 8 wells. The positive samples in cell cultures were tested after a positive cytopathic effect. The reaction was performed in a slide with two holes: 50 μl of VIRUS SCREEN (Chemicon) was added in one ("pool" of 7 monoclonal antibodies) and 50 μl of normal mouse serum in other (negative control). After incubation at 37 °C and washing with PBS and Tween 20 solution, it was added 50 ml anti-mouse conjugate with Fluorescein Isotiocyanate (FITC) and the slides were read in the immunofluorescence microscope24. Positive samples were evaluated for viral identification, applying the reaction with specific monoclonal antibodies anti-RSV (MAB 858-4), anti-influenza A (MAB 8251), anti-influenza B (MAB 8661), anti parainfluenza 1 (MAB 834-3), anti-parainfluenza 2 (MAB 844-3) and anti-adenovirus (MAB 805) and others, as described previously.
This protocol was submitted and approved by Ethical Committee from Hospital das Clínicas, Faculty of Medicine, University of São Paulo. The samples were obtained with the patients or controls and/or the parents approval.
Statistical analysis was applied (mean and standard deviation) and the comparison between two proportions, means, Fisher and Wilcoxon test were developed considering the established hypothesis.
RESULTS
Considering clinical data from group I, 66.7 % had presented wheezing before 2 years old, 66.7 % reported positive familial history for atopy and 82,9 % were associated to allergic rhinitis. URTI were referred as triggering attacks in 94.3 %, with sazonal occurrence of the crisis in 68.6 %, worsening during the winter in 92.8 % and during the summer in 7.2 %. The severity of asthma in group I was classified as: severe (42.9 %), moderate (25.7 %) and mild (31.4 %).
Acute asthma attack in G I children initiated 3.7 ± 2.5 days before the day of sample collection, and the URTI symptoms, in the same children initiated 3.8 ± 2.6 days before the day of collection, without statistical difference between them. In group I, 86.1 % of the children used bronchodilators at the moment of samples' collection; in group II, only 50 % of the children whom presented attacks were using bronchodilator medications of short effect and this difference was significant (p < 0.05). Corticosteroids were not used in 15/36 in group I and 7/18 patients in group II; sporadic use of inhaled steroids were referred in 11/36 of group I and 4/18 patients of group II. None of the individuals in groups III and IV were medicated with steroids or bronchodilators. The peak flow values in group I children, asthmatics with URTI were significantly higher (p < 0.05) than the values obtained in children of group II, asthmatics without URTI (11/18). In group IV, control, the children haven't presented clinical symptoms of URTI. None of the children were hospitalized. Detailed clinical data are described in table I.
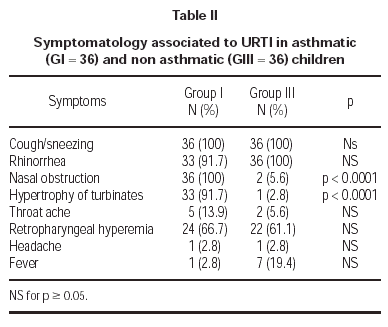
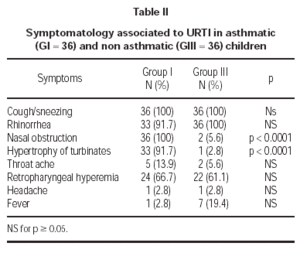
Symptomatology reported during URTI in asthmatics (G I) and non asthmatics (G III) are described in table II. Nasal obstruction and hypertrophy of turbinates were more accentuated in asthmatics (G I).
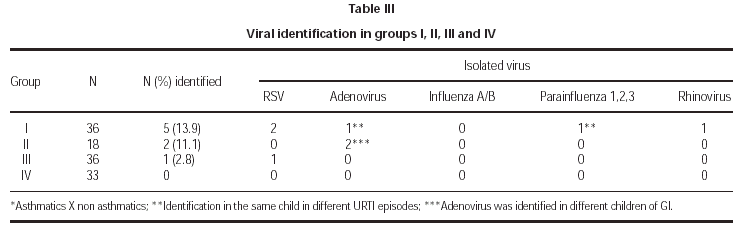
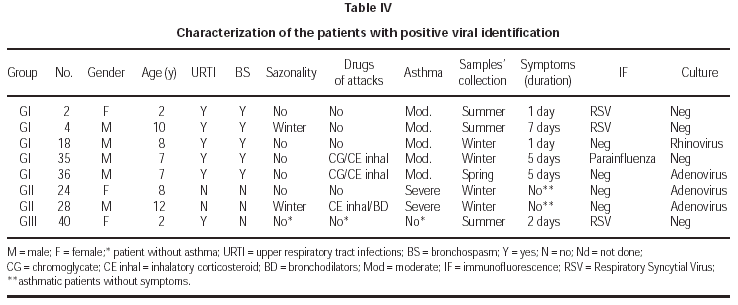
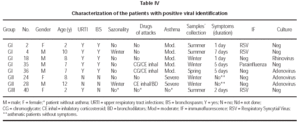
Viral identification showed positivity in 5/36 (13.9 %) of asthmatic children during asthma attacks (G I), 2/18 (11.1 %) of asthmatics without acute attacks; 1/36 of children with URTI. There was no positivity of viral isolation in the control group (G IV). The samples were collected during the winter time in 84 % and the positive identification occurred in this period for 4/8 samples. The following virus were isolated: RSV (2), Adenovirus (1), Parainfluenza (1) and Rhinovirus (1) in G I; Adenovirus (2) in G II and RSV (1) in G III (table III). Two patients (G I, G III) had viral identification at 2 years of age and RSV was implicated. In two patients without evidence of URTI (G II), adenovirus was isolated. The severity of asthma was moderate in 5/7 and severe in 2/7 patients and sazonality of asthma attacks was not referred in 5/7 patients with positivity to viral infections. Immunofluorescence identified 50 % of the infectious agents and the other 50 % were identified after culture. Detailed data about patients with positive viral isolation are presented in table IV.
DISCUSSION
The aim of this study was to evaluate the relationship between virus and asthma attacks in pediatric group, with special reference to children above 2 years of age. Most of the papers refer to RSV and rhinovirus as a cause of asthma exacerbations. We focus the diagnosis in other virus as inducers of asthma attacks as well as their role in older children. Four groups were included, asthmatics (G I and G II) and non asthmatics with and without URTI (G III and G IV). The higher rate of viral detection occurred in group I and no virus was isolated in non asthmatic children without URTI (G IV). Viral identification in asthmatic and non asthmatic children with URTI (G I and G III) was 8.3 %, low in comparison with previous studies3,4,6,10,11,25. In children without URTI symptoms (G II and G IV), viral detection occurred in 3.9 %, compatible with other studies10,11,25,26. Considering symptomatic asthmatic children (G I), the rate of viral identification was low (13.9 %), nevertheless, significantly higher than non asthmatic children with URTI.
Our results could be influenced by several factors: the strict criteria for URTI, inclusion of non hospitalized children only, restriction of the protocol to older children and application of conventional techniques of viral identification.
The initial difficulty was related to the diagnosis of URTI in asthmatic children, most of them presenting allergic rhinitis. We considered three or more symptoms or related signs of URTI to establish the diagnosis. The limit of seven days with symptoms was established in order to reduce concomitant bacterial infection. Previous studies used as diagnostic criteria only one or two symptoms, including cough and/or wheezing and, frequently, the only evidence of URTI was the patient's report2,6,8,27,28,29. Asthmatic children during URTI (G I) presented nasal obstruction and hypertrophy of turbinates with higher frequency than non asthmatics (G III), possibly as a consequence of rhinitis.
The symptoms of asthma attacks were referred simultaneously with URTI in G I. Mertsola et al (1991) observed the development of bronchospasm only 48 hours after the beginning of the infection9. Another observation was that the severity of the acute attacks in hospitalized patients or evaluated in emergency rooms was related to higher rates of viral identification2,3,9,11,19,26. Our study evaluated patients with attacks of mild or moderate intensity, including recent beginning (up to 3 days) and these factors probably influenced the low viral identification.
Children under the age of 5 years have the highest hospitalization rate for asthma. It is more than twice as high as in children 5 to 10 years of age and 5 times higher than in older adolescents and young adults21. There was a predominance of children between 5 and 10 years of age in all groups, with a mean of 7.3 years, above the observed range in other studies (2,4,9,27). Our findings were comparable to previous studies developed in asthmatic adults, suggesting that children with higher age could follow the same behaviour as adults in relation to the presence of virus as triggering factor fo the attacks18,28,30.
The samples were collected after 3,8 days (mean) from the beginning of the symptoms and in 13/36 cases of group I, the material was collected after the third day of URTI. It is reported that viral isolation progressively decreases after 48 hours from the beginning of the clinical symptoms26,31. Freymuth et al (1999) detected viruses in 33,3 % of nasal aspirates using conventional techniques11. The combination with molecular technique improved the detection to 81,8 % of the samples. It is also important to underline that polymerase chain reaction (PCR) is theoretically unable to differentiate a genuine respiratory infection from a long term carriage or a latent infection by the virus11. Recently, Simpson et al (2003) described the combination of induced sputum and reverse-transcription polymerase chain reaction (RT-PCR) in the detection of respiratory pathogens32. This methodology was not available during this protocol.
Rhinovirus is the most frequent cause of common cold. This virus is isolated in cultures with HeLa-I, but replication is better in diploid cells of human fibroblasts. Previous studies of upper respiratory infections have suggested that detection with cell cultures missed many infections, mainly rhinoviruses33. We performed two additional passages in cell cultures if previous negative results were found. It has been described that several rhinoviruses are slow growing in cultures34.
Adenovirus was detected in one child of group I and in two children of group II. It is well known that adenovirus is associated to latent or persistent viral infection, specially in a carrier of chronic respiratory disease35,36, and it could also be present in individuals with asymptomatic infection.
In the present study, respiratory syncytial virus was detected in three children, two of them asthmatics (group I) and one non asthmatic (group III). One of the asthmatic children with RSV identified was 10 years old, different from other reports that isolated this virus in younger children2,27,37,38. The other two children with RSV were 2 years old. Henderson et al (1979) observed that 98 percent of children attending day care during their first year of life became infected with RSV and 74 percent were reinfected during their second year20,39. Glezen et al (1986) reported that in the school age years, the frequency of lower respiratory tract involvement wih RSV infection remains appreciable40.
Parainfluenza virus was only detected in one case of group I, and it was associated with asthma attack in older children, as referred previously2,3,6,9,19. In this child with parainfluenza virus, adenovirus was detected one month later, when asymptomatic, demonstrating the possibility of subsequent viral infections, associated or not with acute attack, causing prolonged symptomatology sometimes. The parainfluenza viruses cause a spectrum of respiratory illnesses similar to those caused by RSV, but result in fewer hospitalizations19.
Influenza virus has been related to acute attacks of asthma in younger chidren and with mild manifestations; with higher frequency in asthmatics than non asthmatics2. Although the adequate methodology for identification and the predisposal for this age range in children, influenza was not detected. Identification rate in previous studies were variable, from zero to approximately 40 %3,4,6,7,9; usually, the higher rates were obtained in more severe clinical manifestations8.
In summary, the rate of viral detection was higher in asthmatic children than non asthmatics, symptomatic or not, suggesting a possible susceptibility to viral infections. They could also be a triggering factor of the attacks, although it could not be the most preponderant in older children. In addition, these findings of morbidity could also influence immunization schedule established for the specific ages and the occurrence of underlying conditions.
ACKNOWLEDGEMENTS
The authors would like to acknowledge the Dept of Pediatrics, Faculty of Medicine, University of São Paulo for referring the patients.