INTRODUCTION
Cancer and its treatment are an important cause of secondary immunodeficiency in childhood1,2.
Leukaemias constitute the most frequent malignancies in the pediatric age (30 % of all neoplastic diseases3,4). In 95-98 %3 of patients leukaemias are acute, and of these 75-80 %3,4 correspond to acute lymphoblastic leukaemias (ALL) and the rest to acute myeloid leukaemias (AML).
The majority of children diagnosed with leukaemia don't have a previous immunodeficiency1,5. However, the development of leukaemia itself is an evidence that there has been a failure in their immunity system1. Immune disorders associated with cancer are varied, and quantitative and qualitative changes have been described1. Among those quantitative stand out: decrease of lymphocytes number, decrease of delayed hypersensitivity, decrease of mitogen responses, decrease of immunoglobulins (Ig) synthesis6,7, decrease of monocyte oxidative responses, decrease of cytokine responses, and increase of monocyte suppressor activity. Regarding qualitative changes stand out the deficiencies in chemotaxis, phagocytosis and bactericidal activity.
On the other hand, immune changes in these children are also secondary to the treatment they receive2,5,8: surgery (breakdown of skin and mucosal integrity), radiotherapy (lymphopenia), chemotherapy (granulocytopenia) and immunosuppressive treatments, such as steroids, that modify antiinflammatory responses. Another important factor in these children's immunodeficiency is malnutrition5,9.
This study seeks to demonstrate that cancer and antineoplastic treatment cause a defect in humoral immunity in pediatric patients with ALL, and that this immunodeficiency is potentially reversible and transitory, but it can have an influence on disease's evolution and outcome in some patients.
MATERIAL AND METHODS
A retrospective study of all patients diagnosed with ALL in our hospital and treated according to the SHOP-LAL-94 protocol (table I) has been carried out. This protocol was used from April 1994 until August 1999. All patients have been treated and followed up in our center.
Patients have been classified according to the therapeutical protocol followed in three groups: patients with ALL cell line B (ALL-B), patients with ALL-B that suffered a relapse of their disease, and patients with ALL cell line T (ALL-T). The first group has been treated with the SHOP-LAL-94 protocol. Patients in the second group have followed SHOP-LAL-94 protocol at leukaemia's onset, and the SHOP protocol valid during the relapse's year at relapse. Patients with ALL-T have followed a treatment with an induction stage based on SHOP-LAL-94 protocol and the rest of treatment according to non-B-NHL protocol.
Patients with congenital immunodeficiencies, acquired immunodeficiencies, Down syndrome and/or immunosuppressive treatments previous to ALL diagnosis have been excluded. Also those patients treated totally or partially in other centers have been excluded.
A retrospective study of the selected patients has been carried out by clinical and laboratory data review. Epidemiological data regarding gender, age, year and season at diagnosis, personal and family antecedents, type of treatment received and clinical evolution of patients have been collected. As for analytic data, we have collected the following: IgG, IgA and IgM concentration (measurement by nephelometry, Behring®), total leukocyte number, manual or automatic leukocyte formula (determination by hematologic counter Cobas ABX®), and absolute number of CD4 lymphocytes (determination by flow cytometry, FACS alibur®). Laboratory data have been analysed at malignancy's onset, during the antineoplastic treatment period, and up to 12 months after finishing treatment or until immunoglobulins' normalization. In the analytic data collection we have taken under consideration that some patients with persistent and severe immunoglobulins' deficiency have received endovenous treatment with gammaglobuline.
For statistical analysis the computer program Statistical Package for Social Science (SPSS inc., Chicago, Ill) has been used in its 10.0 version for Windows. Quantitative variables were compared using the Student's t test. Results were considered significative when p < 0.05.
RESULTS
Epidemiological data
Clinical histories of 55 pediatric patients (0-16 years) diagnosed with ALL in our hospital between April 1994 and August 1999, and that have followed the SHOP-LAL-94 protocol, have been reviewed. Two patients have been excluded because of Down syndrome, other two because of being transfered to other centers to go on with their treatment, and one for having been partially treated in another country. Finally, 50 patients have been included in this study: 44 had ALL-B (88 %) and 6 had ALL-T (12 %).
ALL-B (n = 44 patients)
There were more girls (56.8 %). Median age at disease's onset was 4 years and 10 months, with a range from a newborn with congenital leukaemia to 15 years and 10 months. There were 9 cases in 1994, 6 in 1995, 7 in 1996, 11 in 1997, 6 in 1998 and 5 in 1999. There were more cases in autumn (27.3 %) and summer (27.3 %), without significative differences.
Only 7 patients had personal infectious antecedents: repeated otitis media acuta, repeated pharyngo-amygdalitis, chickenpox, infectious mononucleosis, cellulitis, pneumonia and meningococcal sepsis. There were family antecents related to the disease in 7 cases: family neoplastic diseases in 6 children, and chronic neutropenia in the father of the newborn with congenital leukaemia.
59 % of patients had high risk criterion (table II). All patients had a port-a-cath (PAC) and received treatment with chemotherapy and steroids according to the SHOP-LAL-94 protocol.
Evolution was favourable in 35 cases (79.5 %). There were 7 patients that suffered relapse of their malignancy (15.9 %) and 2 died (4.5 %), one of them the newborn mentioned, that had a disseminated intravascular coagulation with refractary hipotension at seven days of age (6 days after beginning chemotherapy), and a six-month-old patient with a septic shock by Proteus mirabilis one month after beginning chemotherapy.
Relapse of ALL-B (n = 7 patients)
There were five girls (71.4 %) and two boys. At the malignancy's onset two patients had low risk criterion and five had high risk factors (table II). All had a PAC and followed SHOP-LAL-94 protocol at the disease's onset. Three patients suffered a precocious relapse and four a late relapse (table III). In the relapse moment patients were included in the SHOP protocol valid in that year. Three children had a bone marrow transplantation (BMT), one was autologous and the other two were HLA-identical allogenic, with conditioning that included total body irradiation (TBI) or intensive chemotherapy. Five patients died (71.4 %), including the three patients that had had BMT.
ALL-T (n = 6 patients)
There were five boys (83.3 %) and only one girl. Median age at disease's onset was 6 years and 6 months. There were more cases in autumn (3 patients), without significant differences. None of the patients had personal or family antecedents related to the disease.
A patient died three days after diagnosis due to an irrecoverable cardio-respiratory arrest. The other patients had a PAC and were treated with an induction stage based on SHOP-LAL-94 protocol and the rest of the treatment according to non-B-NHL protocol, which includes polichemotherapy, steroids, BMT and craneal radiotherapy.
There were three patients that relapsed: two of them one month after BMT, and another one year after BMT. All of them died. Two patients have followed a favourable evolution until nowadays.
Humoral immunity: immunoglobulins' concentration
ALL-B
At the leukaemia's onset all patients, but the newborn with congenital leukaemia, had normal immunoglobulins' concentration.
Immunoglobulin G (fig. 1) was normal in all patients at the malignancy's onset, with a median value of 9060.40 +/2848.00 mg/L. During treatment, its concentration decreased to pathologic values in 53 % of patients. Decrease during treatment period was significant, with a median value of 4095.10 +/2325.83 mg/L (p = 0.006).
Figure 1.--IgG's evolution in patients affected by ALL with a favourable outcome.
At the onset immunoglobulin A (fig. 2) had normal concentration in all patients, except for the newborn with congenital leukaemia (physiological IgA absence), with a median of 884.60 +/485.83 mg/L. During treatment period it decreased to pathologic values in 29 % of patients. This decrease was also significant, with a median concentration of 356.60 +/249.42 mg/L (p = 0.008).
Figure 2.--IgA's evolution in patients affected by ALL with a favourable outcome.
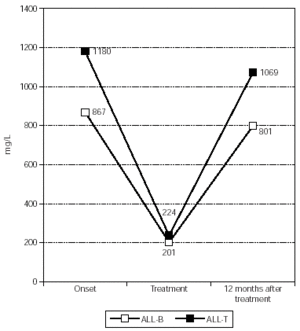
As for immunoglobulin M (fig. 3), at the onset it was normal in all patients, except for the newborn mentioned (pathological IgM absence), and its median concentration was 867.20 +/439.78 mg/L. During treatment IgM decreased to pathologic values in 65 % of patients and also this decrease was significant, with a median of 201.10 +/47.75 mg/L (p = 0.006).
Figure 3.--IgM's evolution in patients affected by ALL with a favourable outcome.
Median time for Ig'concentration normalization was 12 months after treatment in patients with a favourable evolution (absence of relapse and/or death). Nowadays two patients have a persistent IgM deficit 14 and 15 months after treatment, another has an IgG deficit 13 months after treatment, and another has an IgM and IgG deficit 15 months after treatment, in spite of a good clinical outcome until now.
Relapse of ALL-B
All patients had had normal Ig'concentration at their leukaemia's onset. A patient had a persistent IgM deficit from the treatment's end until relapse, another had an IgM decrease at relapse, and another had global Ig'concentration decrease at relapse. The other four patients had normal Ig' concentration at relapse.
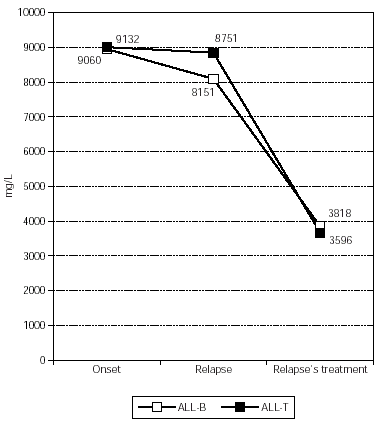
IgG (fig. 4) had a median concentration of 8150.57 +/1837.99 mg/L at relapse and during relapse's treatment it decreased significantly in all patients, with a median of 3817.60 +/1709.22 mg/L (p = 0.009).
Figure 4.--IgG's evolution in patients affected by ALL with relapse.
As for IgA (fig. 5), at relapse it had a median value of 900.00 +/535.63 mgU/L and during relapse's treatment it decreased to pathologic values in 29 % of patients, with a median concentration of 622.00 +/453.57 mg/L. This decrease was not statistically significant.
Figure 5.--IgA's evolution in patients affected by ALL with relapse.
Finally, IgM (fig. 6) had a median concentration of 468.71 + /263.84 mg/L at relapse and it decreased to patholocic values in all patients during relapse's treatment, with a median of 316.40 + /107.96 mg/L, which was not statistically significant.
Figure 6.--IgM's evolution in patients affected by ALL with relapse.
All five patients that died had IgM absence (concentration < 186 mg/L) during the days previous to death, and three of them had global Ig' concentration decrease before death. The two survivors have a persistent IgG and IgM deficit until now, four months after relapse.
ALL-T
Analytic data refer to five patients, as one patient died three days after diagnosis. At the malignancy's onset all patients had normal Ig' concentration.
As for the two patients with a favourable outcome, IgG (fig. 1) had a median of 12,503.50 +/3373.61 mg/L at onset, and it decreased to pathologic values in both patients during treatment, with a median of 4320.50 +/296.28 mg/L. Ig A (fig. 2) had a median of 2778.00 +/-2341.94 mg/L at the onset and during treatment it decreased pathologically in both of them with a median value of 919.50 +/795.50 mg/L. Finally, IgM (fig. 3) had a median concentration of 1180.00 +/193.75 mg/L at onset and it decreased significantly in both patients during their treatment with a median of 223.50 +/53.03 mg/L (p = 0.014).These two patients had normal Ig concentration 6 months after treatment.
Regarding the three patients with relapse, one patient had normal Ig'concentration at relapse, while the other two had a deficit in IgG and IgM. IgG (fig. 4) had a median of 9132.33 +/2733.79 mg/L at the onset, 8751.33 +/5541.73 mg/L at the relapse, and 3595.67 +/741.12 mg/L during treatment. IgA (fig. 5) had a median of 1313.00 +/993.90 mg/L at the onset, 681.33 +/242.92 mg/L at the relapse, and 254.67 +/108.24 mg/L during treatment. Finally, IgM (fig. 6) had a median of 1264.67 +/650.38 mg/L at the onset, 532.00 +/244.35 mg/L at the relapse, and 284.33 +/173.79 mg/L during treatment. All Ig decreased during relapse's treatment, without statistical significance. All these patients died and had a global Ig deficit before death.
DISCUSSION
As for epidemiological data of patients with ALL in our hospital, there is a predominance of ALL-B in girls and of ALL-T in boys, in contrast to that described in literature, with a global ALL incidence somewhat higher in boys than in girls (1.2:1)3,10,11. Incidence of ALL-B is higher in children with age between 3 and 5 years, while ALL-T appears in older children3,11. With the collected data it can be said that usually patients don't have personal or family antecedents related to the disease, and that there is not a seasonal predominance in ALL onset.
As it is written in literature, ALL-T has a worse prognosis than ALL-B3,10,11, with a relapse index in our series of 60 % versus 16 %. That's why treatment in ALL-T needs to be more intense and aggressive.
Regarding the immune system, it is important to remember that patients with cancer have a wide variety of defects, which affect both cellular and humoral immunity1,2,8. One of the most obvious defects is the fact that the tumor itself has grown without an appropriate immune response to stop it1. On the other hand, antineoplastic treatment also contributes to immunodeficiency in these patients, rising their susceptibility to infections2,8. Viral and bacterial infections are an important cause of morbimortality in patients with malignancies2,5,8.
In our research we have studied Ig' concentration from ALL's diagnosis and through disease's evolution. It has been seen that at leukaemia's onset Ig'concentration was normal in all patients, except for the newborn with congenital leukaemia, who had an IgM's absence. As it is said in literature, children diagnosed with ALL have a normal IgG, IgA and IgM's concentration at disease's onset4,8. In contrast with patients affected by lymphoma, children with ALL maintain a normal cellular and humoral immunity until they receive an intense antineoplastic treatment or they reach the final stage of their disease5.
However, during treatment the majority of patients suffer a decrease in Ig'concentration4,8. In our series there are no significant differences in Ig's concentration evolution between patients with ALL-B and those with ALL-T, as it is seen in figures 1-6. In patients affected by ALL-B with a favourable evolution there is a significant decrease in all Ig during treatment (p = 0.006 for IgG, p = 0.008 for IgA and p = 0.006 for IgM). Ig' decrease in the two patients affected by ALL-T with a favourable evolution is only significant for IgM (p = 0.014) probably because of the small number of patients in the group.
As for patients with relapse of their malignancy, there is a significant decrease in IgM's cocentration at relapse's moment (p = 0.047). During relapse's treatment there is a decrease in all Ig'concentration, which is significant only for IgG in patients with relapse of ALL-B. IgM at relapse's moment is already very low, that's why the decrease during treatment results no significative. There are no significant decreases during relapse's treatment of ALL-T probably because of the fact that there are few patients. It must be pointed out that the only two survivors affected by relapse of their malignancy (ALL-B) have a persistent deficit in IgG and IgM's concentration four months after relapse.
It seems that the persistent IgM deficit is correlated with a higher risk of relapse and death, although more studies are needed to confirm this result. IgM was significantly lower in patient's at relapse's moment than at their malignancy's onset (p = 0.047), and 75 % of patients that died had IgM absence (concentration < 186 mg/L) previous to death. On the other hand, IgA is the less affected immunoglobulin in our patients, decreasing only in patients with advanced disease, especially previous to death.
In patients with a favourable evolution of their leukaemia, Ig'concentration decrease is normalized before one year after treatment. In our series patients with ALL-B that had a good outcome had normal Ig'concentration 12 months after having finished their treatment, and the two patients with ALL-T that had a good evolution had normal Ig'values 6 months after treatment.
A deeper knowledge of the immunological disorders associated with cancer and its treatment can contribute to clarify cancer ethio-pathogenic mecanisms, as well as to improve survival and quality of life of children with cancer.
CONCLUSIONS
1. Children diagnosed with ALL have normal IgG, IgA and IgM concentration at their disease's onset.
2. During treatment the majority of patients suffer a decrease in the concentration of one or more immunoglobulins.
3. IgG and IgM are the most affected immunoglobulins in our series both in ALL-B and ALL-T.
4. A persistent deficit in IgM is associated in our study with an increase in the risk of relapse and death.
5. IgA is the less affected immunoglobulin in ALL, decreasing only in patients with advanced disease, especially previous to death.
6. In patients with a favourable evolution immunoglobulins normalize before one year after treatment.
ABBREVIATIONS
ALL Acute Lymphoblastic Leukaemia
SHOP Sociedad de Hemato-Oncología Pediátrica/ Pediatric Hemato-Oncology Society
Ig Immunoglobulins
IgG Immunoglobulin G
IgM Immunoglobulin M
IgA Immunoglobulin A
NHL Non-Hodgkin Lymphoma
PAC Port-a-cath
BM Bone Marrow
BMT Bone Marrow Transplantation
CNS Central Nervous System