Profilin is a panallergen contained in pollen, plant foods and latex. Although cross-reactivity is expected while performing skin prick tests (SPT) with allergens that contain profilin, this is not always noticed. The purpose of this study was to detect if profilin is contained in the commercial SPT extracts of pollen and plant foods which, in their fresh form, contain determined epitopes of profilin.
Material and methodsCommercial SPT extracts of different pharmaceuticals were analyzed using sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). The study included purified palm date profilin, peach (whole, pulp and peel extracts), hazelnut, Olea europea, Parietaria judaica and Phleum pratense.
ResultsProfilin was detected in all, but peach extracts; it was neither contained in the whole peach extract nor in the ones of peel or pulp.
ConclusionThe only accurate way to detect sensitization to profilin, while performing SPT, is the use of purified profilin extract. Even if a plant food or pollen contain an identified molecule of profilin, the relevant SPT commercial extract may not.
Profilins are panallergens found in pollen, plant foods and latex.1 The initial sensitization to them is usually induced by inhalation of pollen and the most usual clinical expressions they are causing are allergic rhinitis and oral allergy syndrome (OAS).1–3 Although cross-reactions between pollen and plant food profilins are often noticed, this does not happen with all of them; in some cases only partial cross-reactivity between profilins of different origin exists, while there are species-specific IgE epitopes on various profilins not causing cross-allergies.4–6
In various regions the prevalence of sensitization to profilin depends on the local flora, since pollen induces it. In a recent study we determined the prevalence of sensitization to profilin in Greece and compared the SPT results of profilin (commercial extract of purified palm tree profilin Pho d 2) with those of pollen (Phleum, Parietaria, Olive) and plant foods that – in their raw form – contain molecules of profilin.7 Sensitization to profilin was detected in 29 out of 264 atopic patients.7 However, comparing positive SPT to pollen and food allergens with the positive SPT to profilin, no statistically significant correlation was demonstrated.7
Based on the assumption that profilin was contained in all SPT extracts of the study (as it happens in their raw form), a correlation of profilin SPT results with the tested allergens, was expected. The purpose of this study was to detect if profilin is contained in the used pollen and food commercial extracts; its lack would explain the noticed contradiction. The method of sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) was used for this purpose.
Material and methodsSPT extractsThe presence of profilin was examined in SPT extracts that were used in the original study; whole peach (Alyostal, Stallergens, France; 1000IC/ml), peach pulp and peach peel separately (LETI, Spain; 5μg/ml), hazelnut, Olea europea, Parietaria judaica and Phleum pratense (Allergy Therapeutics, UK; 10,000DU/ml). Purified SPT extract of palm date profilin (ALK-Abello, Denmark; 50μg/ml) was also analyzed.
SDS-PAGE visualization of profilinSDS-PAGE is a method used for the separation of protein molecules, based on their molecular weight.8 Following this method, the tertiary structure of a protein is reduced to a linear molecule, using agents that reduce it, as well as SDS detergent and boiling. Although SDS-PAGE cannot be used to detect profilin's immunologic activity it can be helpful to determine its presence in an extract.
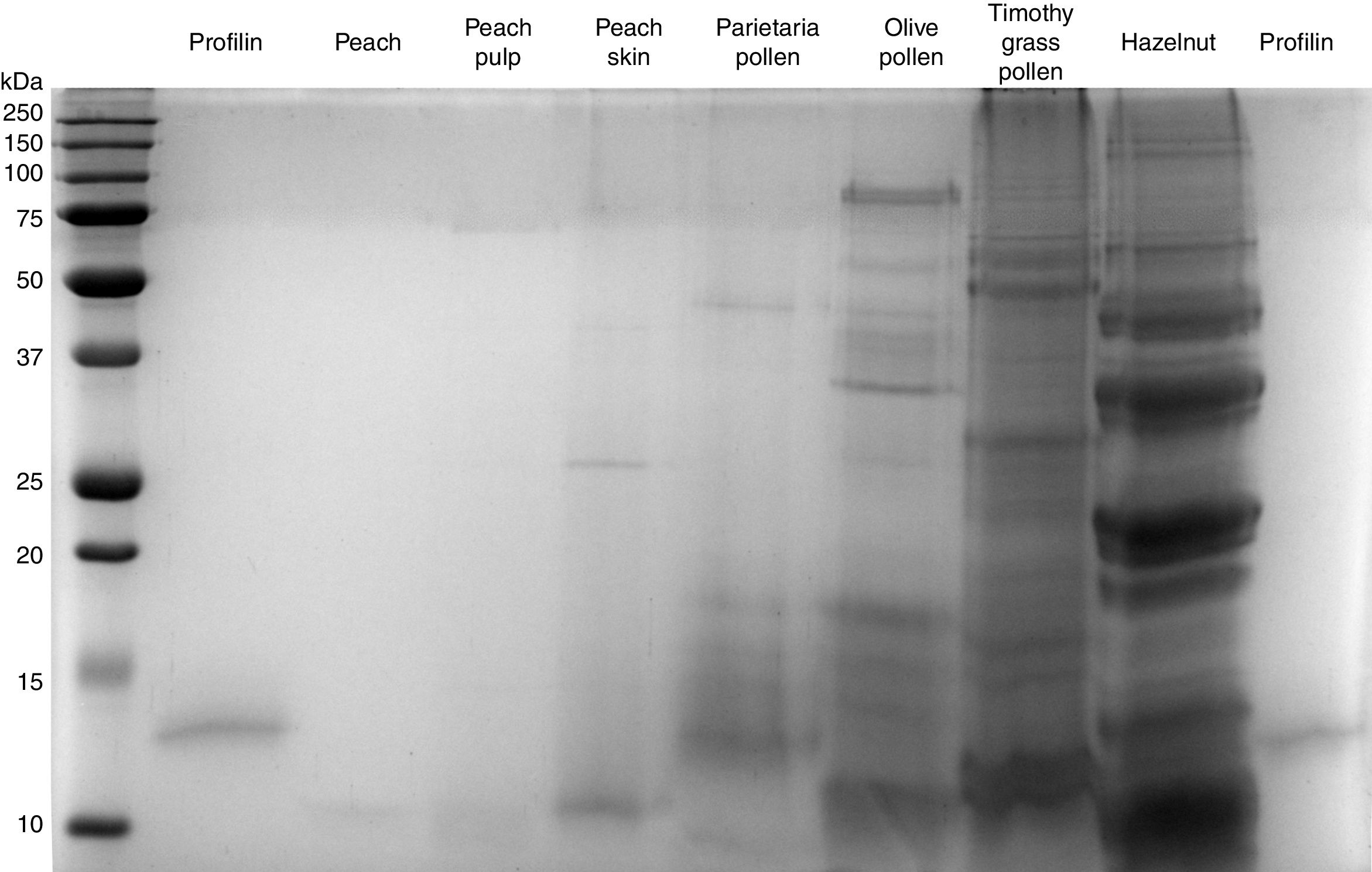
Equal volumes of samples were heat-denatured in 2× SDS Laemli Buffer for 5min in 95°C and 40μl of each sample were loaded and electrophoresed on a 14% SDS-polyacrylamide gel together with a standards ladder (Precision Plus Protein All Blue Standards – Biorad, CA, USA). After completing the run, the gel was incubated in Commassie Blue protein staining solution (0.1% Coomassie Brilliant Blue R-250, 50% methanol, 10% glacial acetic acid) for 5min, then washed for 2h in a destaining solution (50% methanol, 12% glacial acetic acid) and finally washed overnight in dH2O. The gel was visualized on a Biorad ChemiDoc System using a standard colorimetric protocol.
ResultsThe presence of profilin was shown in the purified extract, as a “light” protein of about 13kDa (Fig. 1). Profilin was detected in hazelnut, Phleum, Parietaria. It was also detected, but less clearly, in Olea. No profilin was contained in any peach extract; neither in the whole peach extract nor in those of peel and of pulp. Peach SPT extracts under examination were from two different suppliers.
In all three peach extracts the only common protein contained was a “light” one of about 10kDa, corresponding to lipid-transfer protein (LTP). LTP was also contained in the other SPT extracts, with the exception of the purified profilin. SDS-PAGE was performed twice, following the standard method, with the same results. Electrophoresis of purified profilin and peach extracts was repeated skipping the heat process. No new band occurred in the new image (data not shown).
DiscussionThe use of purified profilin (Pho d 2) SPT extract for in vivo detection of sensitization, is considered reliable and the use of Phl p 12 and Pho d 2 SPT extracts’ sensitivity and specificity are similar or higher to those of in vitro detection, with the use of molecular techniques.9,10 Setting the relative purified SPT extract as the standard marker of sensitization to profilin, we have formerly tried to identify a combination of the rest SPT results that would be predictive of profilin sensitization. Only double positivity to Phleum and Olea resulted in having a moderate predictive value, with low sensitivity (69%) and specificity (59.1%).7
Since the ‘Pru p 4’ profilin is a peach protein, it was expected that a whole peach SPT extract would contain it. The results of the current study showed the lack of profilin molecules in the commercial peach extracts, confirming the results of a former study regarding a different peach extract by ALK-Abello S.A.11 This lack explains the discordance between profilin and peach SPT results; peach profilin is not included in the relative SPT extracts, so their use cannot detect profilin sensitization.
SDS-PAGE revealed profilin in the examined SPT commercial extracts of whole hazelnut, P. judaica, O. europea and P. pratense, which respectively contain the described Cor a 2, Par j 3, Ole e 2 and Phl p 12 profilin molecules.12 However, as mentioned before, no statistically significant correlation was found between positive SPT to the purified extract of profilin and the positive SPT of other extracts under examination.7 One of the underlying factors for this discrepancy is that there is only a partial cross-reactivity among different profilins and that their epitopes can be so different that cross-reacting antibodies do not bind to all profilins.6
Another important point is that the SPT extracts of pollen and food allergens consist of a protein mix, so the concentration of profilin in them is lower than in the purified extract. This explains the fact that positive SPT to profilin (wheal of ≥3mm), in most patients, coexists with negative SPT to extracts that contain it in a diluted form.7 However, SPT to a mix containing profilin can result positive in a profilin-sensitive person, especially if one is highly sensitive.
A limitation of our methodology has been SDS-PAGE itself, since profilin is a thermo-labile protein, so minimizing or vanishing peach Pru p 4 could be a drawback. However, in that case other profilins would also be absent. Furthermore, to exclude the hypothesis that Pru p 4 has been included in a minimal dose and that boiling procedure caused its destruction, SDS-PAGE was repeated without boiling; no band appeared in the 12–15kDa area. It should be underlined though, that peach SPT extracts contain LTP that consists in their major allergen and that they are efficient diagnostic tools for food allergy (due to LTP) but not for profilin cross-allergic reactions.13
There is no doubt that SPT with the use of whole allergen extracts is the cornerstone of allergy diagnosis in everyday practice. The use of in vivo and in vitro tests for panallergens, in cases of polysensitization, clarifies patient's allergic profile. Using SPT to panallergens is extremely useful for allergy diagnosis but also a guiding tool before prescribing allergen-specific pollen immunotherapy.14 Nevertheless, sensitivity to a panallergen does not always lead to safe conclusions for the clinician.
Our clinical study had confirmed that SPT sensitivity to profilin is not always related to sensitivity to other allergen extracts, even if they contain it in their raw form.7 Although a single profilin extract sets diagnosis of sensitization, this is not always connected with clinical manifestations. Since allergy to profilin often depends on IgE-binding to species-specific profilin epitopes their detection is useful, as shown in a study that used an inhibition test.15
ConclusionConcluding, our study proved that the only accurate way to detect sensitization to profilin with the use of SPT, is the use of purified profilin extract. Even if a fruit or pollen contains an identified molecule of profilin, the relevant SPT extract may not.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interestThe authors have no conflict of interest to declare.
The authors wish to thank Professor Constantinos Deltas for his support and his contribution in the study's methodology.