Drug provocation tests (DPTs) need technical equipment, staff and time. There are very few allergy centres performing DPTs in Turkey. Therefore many patients are referred to these centres. One day triple–double antibiotic or non-steroidal anti-inflammatory drug (NSAID) oral DPT for determining safe alternatives is safe, cost-effective and time saving compared to conventional one day one drug oral DPT. Our aim was to investigate the safety of antibiotic–NSAID oral DPT performed on the same day to find safe alternatives in multidrug hypersensitive patients.
MethodsForty-two patients who had been diagnosed as having both antibiotic and NSAID hypersensitivity were enrolled to the study between 15 November and 15 July 2010. The reactions were urticaria and/or angio-oedema not including laryngeal oedema for all patients. Two antibiotics–one NSAID or two NSAIDs–one antibiotic triple test have been performed on the same day to study patients (n=22), while the control group (n=20) had taken drugs on three separate days.
ResultsOnly two patients had positive reactions during triple test and two patients had adverse reactions; one had gastric pain, one had nausea. Three patients in the control group had positive reactions. There were no significant differences between the two groups in frequency of adverse and allergic drug reactions (p>0.05). Sixty days were spent for the tests of the control group with only 28 days for the study population.
ConclusionTriple test performed with antibiotic and NSAID on the same day for determining safe alternatives for multidrug hypersensitive patients reporting non-life-threatening allergic reactions seems to be safe and time-saving.
Adverse drug reactions affect more than 7% of the general population.1 In Turkey, the prevalence of self-reported drug hypersensitivity reactions is 11.8%.2 Hypersensitivity reactions to analgesics are the most common, followed by antibiotics2 and antibiotic allergy prevalence is 16.7% in analgesic intolerant patients.3 Multiple drug allergy is rare. However, many individuals do not take only one drug at a time, and receiving antibiotics and analgesics together may increase the risk of adverse drug reactions to both drugs in suspected drug allergic patients. The drug provocation test (DPT) is the gold standard in the diagnosis of drug allergy and is performed both to establish correct diagnosis as well as to find safe alternatives.4 Individual protocols are used and if several drugs are suspected, each is tested in sequence. There is no national standard protocol for DPTs in Turkey and various protocols are used in different allergy clinics for this purpose, and the duration of the test for one drug differs from a few hours to a few days.4
DPTs need technical equipment, staff, time and experience for performing and interpreting test procedure. Since there are very few allergy centres performing DPTs in Turkey, a large number of patients are referred to these centres and our clinic is one of them; generally, two-thirds of suspected drug allergy patients are referred to our clinic from other cities of Turkey. Single blind oral DPTs have been performed in our clinic since 1991 and we have been performing triple or double tests for determining safe alternatives in analgesic and antibiotic hypersensitive patients since 2002 and 2005, respectively. Our clinical practice has shown that one day triple–double antibiotic or analgesic oral DPTs for determining safe alternatives are safe, cost-effective and time saving compared to conventional one day one drug oral DPTs.5,6 Our aim was to assess the risk–benefit ratio of our triple test approach consisting of two antibiotics–one NSAID or two NSAIDs–one antibiotic oral DPT performed on the same day to find safe alternatives in multidrug hypersensitive patients.
MethodsPatientsNinety-four patients who had both suspected analgesic and antibiotic hypersensitivity were enrolled to the study between 15 November and 15 July 2010. Patients who reported non-life-threatening allergic reactions including immediate urticaria and/or angio-oedema were included and patients who had severe allergic-immunological reactions including laryngeal oedema, asthma attack, anaphylaxis, immunocytotoxic reactions, vasculitic syndromes, exfoliative dermatitis, erythema multiforme major/Stevens–Johnson syndrome, drug-induced hypersensitivity reactions (with eosinophilia)/DRESS and toxic epidermal necrolysis were excluded. The diagnosis of drug hypersensitivity was determined by history and/or skin/provocation tests as described in the position papers by the ENDA/EAACI.4,7,8,9 Forty-two patients met the inclusion criteria and patients were divided into two groups. Triple tests have been performed on the same day to study patients (n=22), while the control group (n=20) had taken NSAIDs or antibiotics on three separate days.
Oral drug provocation testsSingle blind oral DPTs were performed for determining safe alternative antibiotics and analgesics. Patients met the following criteria before oral DPTs: absence of an urticaria or angio-oedema attack in the last week; stable asthma (forced expiratory volume in one second at least 70% of the predicted value), and continuation of normal asthma treatment, if the patient had asthma. Criteria for exclusion were history of treatment with short-acting antihistamines during the last 24h, long-acting antihistamines up to 10 days and beta-blockers in 48h before the test; any cardiac, haematologic, renal and gastrointestinal disorder; having one of the other contraindicated drug reactions as mentioned before, and pregnancy or lactation. The time interval between the test and the suspected drug reaction was at least 4–6 weeks. Triple tests are defined as performing the oral provocation tests with two antibiotics–one NSAID or two NSAIDs–one antibiotic consecutively on the same day. DPTs were performed on three separate days in the control group and similar drugs were used for safe alternatives. These tests were performed in our outpatient clinic under careful observation by an allergist and resuscitation facilities were available for emergencies. The patients had a breakfast with a cheese sandwich and then drugs were administered between 9:00 and 12:00 a.m. All the patients were examined, and their basal peak expiratory flow, pulse and blood pressure values were recorded before the test. The drugs were applied in 30min intervals. The patients had a lunch with cheese sandwich and water and were observed until 17:00. The test was ended when the patient tolerated the planned number and doses of tested oral antibiotics and analgesics, or when any reaction occurred. If the test was positive, each drug was administered one by one on three separate days. If the patients had the side effect of vomiting before 12:00 a.m., the tests were also repeated for each drug on separate days. If the decision to repeat the test was taken because of positivity, the repeated test was performed after at least a week. Any possible late phase reactions were evaluated by phone calls 24 and 48h after the test. University ethical committee approved the study and written informed consent was obtained from all participants.
Test agentsThe antibiotics and analgesics used during the triple tests were chosen depending mainly on our experience and also on the data given in the literature on the safe-considered antibiotics and analgesics.4,7,8,9 Roxithromycin, tetracyline, ciprofloxacin, amoxicillin clavulanate, clarithromycin, nimesulide, meloxicam, paracetamol and nabumetone were used in their marketed form. The doses were 500 and 500mg, total 1000mg for clarithromycin, ciprofloxacin, tetracycline; 1000 and 1000mg, total 2000mg for amoxicillin clavulanate; 300 and 300mg, total 600mg for roxithromycin; 100 and 100mg, total 200mg for nimesulide; 7.5 and 7.5mg, total 15mg for meloxicam; 500 and 500mg, total 1000mg for paracetamol and nabumetone.
Positive reactionPositive reaction is defined as bronchospasm (at least 15% drop in the PEF value), naso-ocular reactions (sneezing, nasal discharge, nasal obstruction and conjunctival irritation), urticaria (itching and erythematous lesions raise on the skin), angio-oedema (swelling of the skin and/or external mucosa) and/or systemic anaphylaxis (in addition to urticaria and/or angio-oedema with more than a 30mmHg drop in blood pressure and/or upper airway obstruction). Reactions were classified into two groups: mild reactions were defined as ≤20 urticarial wheals, swelling of mucosa except for larynx, moderate and severe reactions were defined as >20 urticarial wheals, laryngeal oedema, noso-ocular reactions, bronchospasm and anaphylaxis during the challenge procedure.
Calculation of time sparedThe number of days spent for DPTs was calculated with the formula: (number of patients×number of tests). In the case of any drug reaction the tests were repeated separately for the three drugs and the triple test calculation formula was: (number of patients×number of tests)+(number of positive reactions×3).
StatisticsChi-squared tests were used for calculating statistical significance of categorical data. Statistical significance was defined for p values less than 0.05. SPSS MS Windows Release 10.0 was used for statistical analysis.
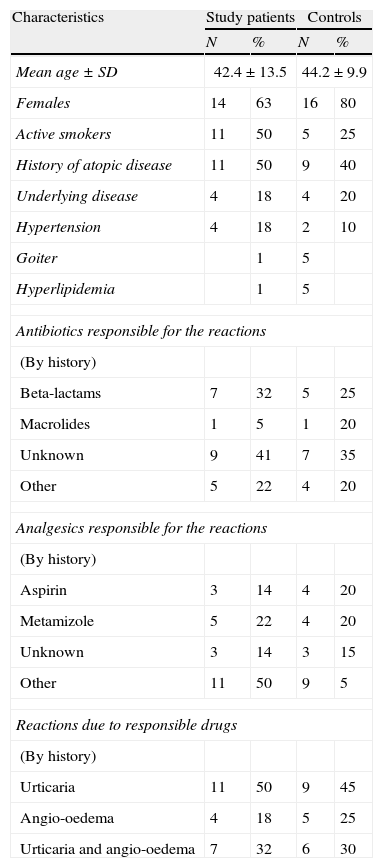
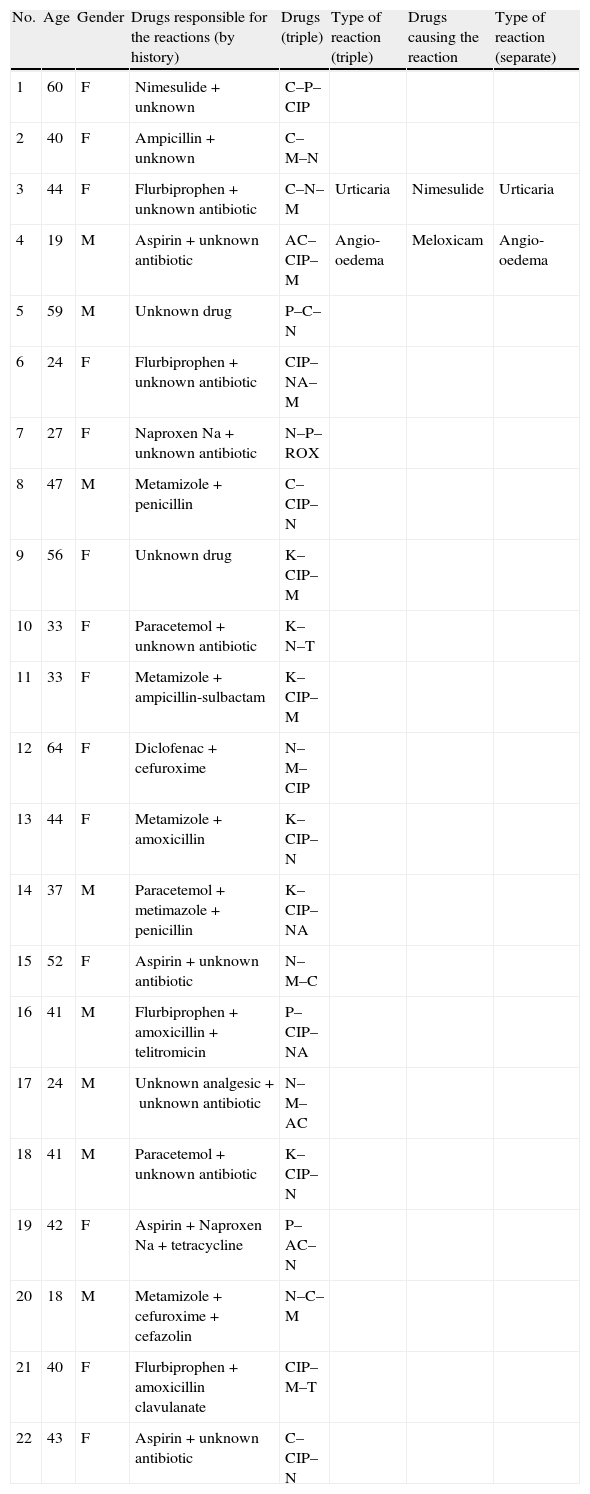
ResultsDemographic and clinical characteristics of the patients are given in Table 1. Twenty study patients reported more than one event with drugs: six subjects two; five subjects three; four subjects four; three subjects six; one subject eight; and one subject ten. Suspected drugs were unknown in two patients. A total of 36.4% were recent events which had happened less than six months before. Two patients had positive reactions during triple test. One patient had urticaria after DPT with nimesulide and the other reaction was angio-oedema due to meloxicam. There was no serious allergic reaction. Two patients had adverse reactions; one had gastric pain due to clarithromycin–ciprofloxacin–meloxicam and the other one had nausea due to paracetamol–nimesulide–clarithromycin combination. All patients tolerated triple test. Results of the positive tests in study population are given in Table 2. Nineteen patients in control group reported more than one event with drugs. Suspected drugs were unknown only in two patients in controls. Total of 10% events had happened less than six months ago in the control group. Three patients in controls had positive reactions. Two reactions were urticaria due to paracetamol and roxithromycin. The other reaction was angio-oedema due to meloxicam. Three patients reported pruritus due to nabumeton, meloxicam and paracetamol. Pruritus resolved in all of them in an hour. So these reactions were not accepted as allergic drug reactions. There were no significant differences between the two groups in the frequency of adverse and allergic drug reactions (p>0.05). Sixty days have been spent for the tests of the control group where it was only 28 days for the study population.
Demographic and clinical characteristic of the patients.
| Characteristics | Study patients | Controls | ||
| N | % | N | % | |
| Mean age±SD | 42.4±13.5 | 44.2±9.9 | ||
| Females | 14 | 63 | 16 | 80 |
| Active smokers | 11 | 50 | 5 | 25 |
| History of atopic disease | 11 | 50 | 9 | 40 |
| Underlying disease | 4 | 18 | 4 | 20 |
| Hypertension | 4 | 18 | 2 | 10 |
| Goiter | 1 | 5 | ||
| Hyperlipidemia | 1 | 5 | ||
| Antibiotics responsible for the reactions | ||||
| (By history) | ||||
| Beta-lactams | 7 | 32 | 5 | 25 |
| Macrolides | 1 | 5 | 1 | 20 |
| Unknown | 9 | 41 | 7 | 35 |
| Other | 5 | 22 | 4 | 20 |
| Analgesics responsible for the reactions | ||||
| (By history) | ||||
| Aspirin | 3 | 14 | 4 | 20 |
| Metamizole | 5 | 22 | 4 | 20 |
| Unknown | 3 | 14 | 3 | 15 |
| Other | 11 | 50 | 9 | 5 |
| Reactions due to responsible drugs | ||||
| (By history) | ||||
| Urticaria | 11 | 50 | 9 | 45 |
| Angio-oedema | 4 | 18 | 5 | 25 |
| Urticaria and angio-oedema | 7 | 32 | 6 | 30 |
Results of the positive tests in the study population.
| No. | Age | Gender | Drugs responsible for the reactions (by history) | Drugs (triple) | Type of reaction (triple) | Drugs causing the reaction | Type of reaction (separate) |
| 1 | 60 | F | Nimesulide+unknown | C–P–CIP | |||
| 2 | 40 | F | Ampicillin+unknown | C–M–N | |||
| 3 | 44 | F | Flurbiprophen+unknown antibiotic | C–N–M | Urticaria | Nimesulide | Urticaria |
| 4 | 19 | M | Aspirin+unknown antibiotic | AC–CIP–M | Angio-oedema | Meloxicam | Angio-oedema |
| 5 | 59 | M | Unknown drug | P–C–N | |||
| 6 | 24 | F | Flurbiprophen+unknown antibiotic | CIP–NA–M | |||
| 7 | 27 | F | Naproxen Na+unknown antibiotic | N–P–ROX | |||
| 8 | 47 | M | Metamizole+penicillin | C–CIP–N | |||
| 9 | 56 | F | Unknown drug | K–CIP–M | |||
| 10 | 33 | F | Paracetemol+unknown antibiotic | K–N–T | |||
| 11 | 33 | F | Metamizole+ampicillin-sulbactam | K–CIP–M | |||
| 12 | 64 | F | Diclofenac+cefuroxime | N–M–CIP | |||
| 13 | 44 | F | Metamizole+amoxicillin | K–CIP–N | |||
| 14 | 37 | M | Paracetemol+metimazole+penicillin | K–CIP–NA | |||
| 15 | 52 | F | Aspirin+unknown antibiotic | N–M–C | |||
| 16 | 41 | M | Flurbiprophen+amoxicillin+telitromicin | P–CIP–NA | |||
| 17 | 24 | M | Unknown analgesic+unknown antibiotic | N–M–AC | |||
| 18 | 41 | M | Paracetemol+unknown antibiotic | K–CIP–N | |||
| 19 | 42 | F | Aspirin+Naproxen Na+tetracycline | P–AC–N | |||
| 20 | 18 | M | Metamizole+cefuroxime+cefazolin | N–C–M | |||
| 21 | 40 | F | Flurbiprophen+amoxicillin clavulanate | CIP–M–T | |||
| 22 | 43 | F | Aspirin+unknown antibiotic | C–CIP–N |
C: clarithromycin; CIP: ciprofloxacin; T: tetracycline; Rox: roxithromycin; AC: amoxicillin clavulanate; N: nimesulide; M: meloxicam; P: paracetemol; Na: nabumetone.
In this study, we assessed the safety of triple test approach for determining safe alternatives in multidrug allergic patients. Our previous reports showed that one day triple–double antibiotic or analgesic oral DPT for determining safe alternatives is safe, cost-effective and time-saving compared to conventional one day one oral DPTs.5,6 Patients who experienced multiple adverse drug reactions reach allergists in stress and they need a quick and simple answer about which drugs they may safely receive. Currently, there are limited data on the mechanisms and responsible drug metabolites for multiple drug reactions and for these reasons; there are also limited diagnostic tests to confirm the diagnosis.10 Using suspected adverse drug reaction history with limited diagnostic testing, individual management approaches can be designed. The most practical approach is to find safe alternatives as quick as possible. Most patients reach the allergists in active dental, upper or lower airway infection state and they need to take an antibiotic and NSAID at once. DPTs need technical equipment, staff and time in addition to the fact that DPTs are performed only in a few centres in Turkey. Triple test approach can speed up the process to find safe alternatives and it seems to be safe and time-saving. In the present study we have shown that, two times more days have been spent for the tests of the controls compared to the study population.
In our study group, seven patients had active dental infection and one had Helicobacter pylori gastritis and they all needed one antibiotic–one NSAID or a combination of two antibiotics. Triple test approach provided to find both the safe antibiotic and NSAID on the same day and to test the safety of analgesic–antibiotic or antibiotic–antibiotic combination on these multiple drug hypersensitive patients.
There were no significant differences between the two groups in the frequency of adverse and allergic drug reactions. In this study, suspected drug reactions were non-life threatening for all patients and the type of reactions with triple test were all mild and similar with patient's history. Two triple tests were positive in study population where the reactions were urticaria and angio-oedema, when the tests were separately repeated the same reactions also occurred. Although there was a time interval of a week between positive triple tests and separately repeated ones, the total time spent for the tests in study group was still less than the time spent for control groups. Among the study group, two patients had adverse reactions; one had gastric pain and the other one had nausea. However, all patients tolerated the combination of antibiotics and analgesic. The side effect rate is 21.9% (7 in 32 antibiotic hypersensitive patients), and 8.3% (10 in 84 analgesic intolerant patients) in our previous surveys, respectively.5,6 The patients had a breakfast with cheese sandwich before the drugs were administered and this could decrease the gastrointestinal side effects rate. In the control group there were no reported gastrointestinal side effects. Although gastrointestinal side effects are mild and acceptable in the study group, these effects may be the limitation of triple test approach. Three patients in the control group reported pruritus which resolved in an hour spontaneously. So these reactions were not accepted as allergic drug reactions and patients did not report any reaction with these safe drugs when they continue to use the drugs after challenge test.
Twenty patients reported more than one event with drugs in the study group and total of 36.4% were recent events. Most of the patients reported the group of the drugs (antibiotic or analgesic) causing the suspected reactions while suspected drugs were unknown in two patients. Many patients are labelled as allergic to many drugs. Thus suspected drug reactions should be confirmed. In our study, we confirmed only three reactions and the other reactions were accepted as multiple adverse drug reactions according to patient's anamnesis. Suspected reactions were not confirmed in the patients not giving the name of suspected drugs. The true multiple drug hypersensitivity rate could be less in our population. Another limitation is the selection bias, because patients groups are not randomly selected. However, this bias probably did not affect the results, because characteristics of the study and control group were similar.
In conclusion, triple test performed with antibiotic and NSAIDs on the same day for determining safe alternatives for multidrug hypersensitive patients reporting non-life-threatening allergic reactions seems to be safe and time-saving. Patients can tolerate antibiotic and analgesic combination tests and this study, in spite of its limitations, will be useful for allergists to find at least two different type antibiotics or two analgesics on the same day in patients reporting mild allergic reactions.
Ethical disclosureProtection of human and animal subjects. The authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Confidentiality of data. The authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consent. The authors must have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence must be in possession of this document.
Conflict of interestThe authors have no conflict of interest to declare.