Good control of allergic rhinitis (AR) in children is desirable because it is associated with diseases such as asthma. The aim of this analysis of the PETRA study was to characterise its diagnosis and treatment in Spanish children.
MethodsData were analysed for paediatric patients (age 5–17 years, inclusive) included in the PETRA study, which included consecutive patients with allergic rhinitis attending respiratory specialists throughout Spain. Demographic information, disease characteristics (duration, severity according to the Allergic Rhinitis and its Impact on Asthma [ARIA] classification), diagnostic procedures, treatments and physicians’ attitudes to treatment were recorded.
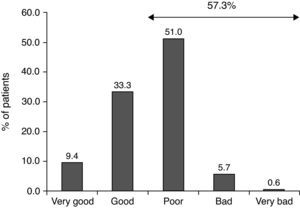
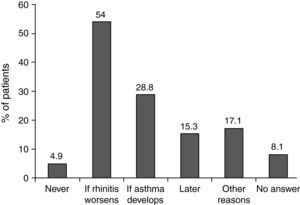
ResultsOf the original sample of 1043 patients, 260 children were included (mean age, 11.7 years; 56.2% boys; 61.9% allergic to house dust mites (HDM) and 38.1% allergic to grass pollen). By ARIA classification, 180/260 (69.4%) had persistent AR and 176/280 (63%) had moderate disease. Asthma was reported in 89/161 (55%) with HDM allergy and 44/99 (45%) with grass pollen allergy. Symptomatic treatment was prescribed in 98.5%, although disease control had been no better than poor in 57.3%. Allergen specific immunotherapy was administered to 56.9%, and was used more often for HDM AR. When asked why specific immunotherapy was not prescribed, two-thirds of the investigators preferred a wait-and-see approach, prescribing immunotherapy if symptoms worsened or asthma developed.
ConclusionsPaediatric patients treated by specialists for allergic rhinitis have moderate or severe disease. Symptomatic treatment was extensively prescribed but often did not achieve good disease control. Many specialists preferred a wait-and-see approach before prescribing immunotherapy.
Allergic rhinitis (AR) is a widespread disease thought to affect about 23% of the general European population aged over 18 years.1 In children, a study conducted in the United States which enrolled healthy children at birth and obtained follow-up data at age six years found that 42% had physician-diagnosed rhinitis.2 As part of the International Study of Asthma and Allergies in Childhood (ISAAC), investigators reported current rhinoconjunctivitis in 1.8–24.2% in children aged 6 or 7 years, and in 1.0–45.1% of those aged 13 or 14.3
As in adults, in children there is evidence for an association between AR and asthma.4,5 Other possible complications include otitis media due to Eustachian tube obstruction6 and hypertrophy with prominence of adenoidal and tonsillar tissue caused by chronic inflammation. These complications may be associated with reduced appetite, delayed growth and obstructive sleep apnoea,7 as well as fatigue and mood changes.8 Allergic rhinitis and associated comorbid conditions can have a negative impact on quality of life9 and have been reported to have a negative impact on school performance.10,11
Treatment is recommended12 and a range of interventions are available, including symptomatic treatment,13 allergen avoidance measures,14 and immunotherapy.15 The PETRA study collected information on the disease characteristics, diagnosis, and treatment of patients attending Spanish specialists. An analysis of the results for the overall population has already been reported.16 Given that the diagnosis and treatment of paediatric patients might be different, we present the results for paediatric patients.
Materials and methodsThe methodology of the PETRA study has already been described.16 Briefly, this was an observational study conducted among specialists randomly selected from public and private clinics. The number of specialists for each province included in the study was weighted to obtain a balanced sample taken from throughout Spain.
The specialists recruited the first four patients to attend their clinic (irrespective of whether they were paediatric patients or not) who met the following inclusion criteria: ≥5 years of age, first consultation (with the investigator), clinical diagnosis of AR (with or without bronchial asthma) due to grass pollen or house dust mites (HDM) and allergic sensitisation confirmed by positive skin prick test and/or grass-pollen specific or Dermatophagoides-specific immunoglobulin (Ig) E greater than 0.70kU/L. The results presented here correspond to the subpopulation aged 17 years or younger.
This was an observational study of normal clinical practice and did not affect the normal prescribing practice of the participating clinicians. The parents or legal guardians gave written informed consent for the data collected on their children to be used in the analysis. Confidentiality of the patients’ data was maintained at all times. The study was evaluated and approved by the ethics committee of the Hospital Clinic, Barcelona, Spain.
Study variablesAt the study visit, sociodemographic and general clinical characteristics of the patients, concomitant treatments and diseases, rhinitis symptoms, diagnostic procedures, and therapies administered for rhinitis were recorded using a standard case report form. The present analysis focuses on data on diagnostic procedures and therapies administered in paediatric patients (aged ≥5 years and less than 18 years). To investigate the procedures used for diagnosing allergic rhinitis, the following data were collected: physical examination, spirometry and lung function tests, imaging studies, and allergy-specific tests (skin-prick tests and specific IgE). To provide information on treatment patterns for allergic rhinitis in paediatric patients in Spain, data were collected on the treatments performed (environmental control, symptomatic treatments, immunotherapy, along with details of each type of therapy). In addition, information on any educational measures was also collected. Rhinitis was classified according to the ARIA classification12 and its modification by Valero et al.17
The investigators filled in a questionnaire about their usual approach to management of patients with AR. This questionnaire included information on the types of treatments that they habitually prescribed in their clinical practice and reasons for prescribing or not prescribing certain treatments.
Statistical analysisDescriptive statistics were calculated for all the patient characteristics recorded during the data-collection visit. These included means and medians with their corresponding standard deviations and interquartile ranges for continuous variables and percentages of the total for categorical variables. Patients with grass-pollen allergy and those with HDM allergy were compared using the χ2 test and Student's t-test, as appropriate. Statistical significance was set at P<0.05.
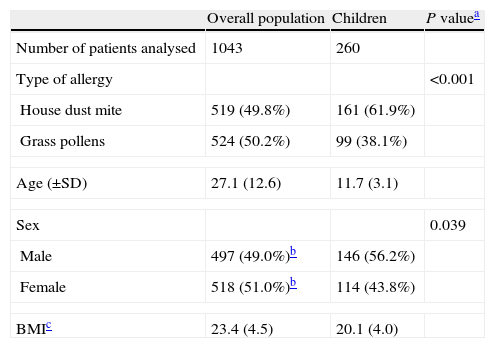
ResultsOf the 1043 patients with analysable data in the overall PETRA study population, 260 were children (age 5–17 years) (Table 1). The mean±SD age of these patients was 11.7±3.1 years and 45% of this paediatric population were aged between 5 and 11 years. Unlike the overall PETRA population, which was balanced in terms of sex, there were more boys (56.2%) than girls (43.8%) in the paediatric population. In addition, unlike the overall population which had roughly equal numbers of patients with HDM and grass pollen allergies, patients with HDM allergies were more strongly represented (61.9%) compared to patients with grass pollen allergies (38.1%).
Characteristics of the overall PETRA study population and the paediatric population analysed in the present study.
| Overall population | Children | P valuea | |
| Number of patients analysed | 1043 | 260 | |
| Type of allergy | <0.001 | ||
| House dust mite | 519 (49.8%) | 161 (61.9%) | |
| Grass pollens | 524 (50.2%) | 99 (38.1%) | |
| Age (±SD) | 27.1 (12.6) | 11.7 (3.1) | |
| Sex | 0.039 | ||
| Male | 497 (49.0%)b | 146 (56.2%) | |
| Female | 518 (51.0%)b | 114 (43.8%) | |
| BMIc | 23.4 (4.5) | 20.1 (4.0) | |
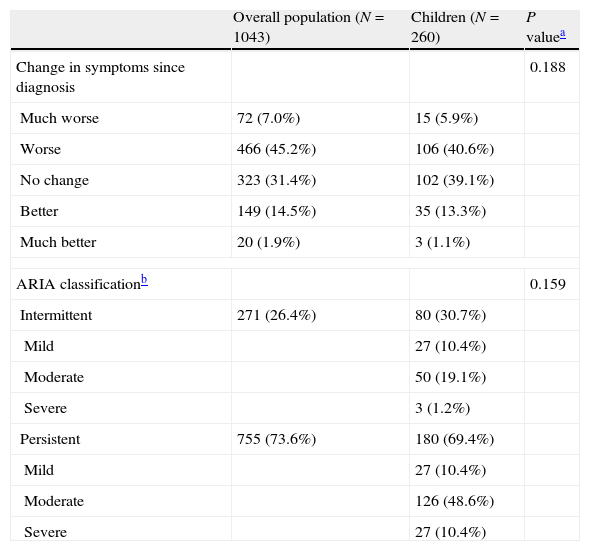
The mean time since onset of first symptoms was four years and 85% reported no improvement in symptoms since initial diagnosis (Table 2). In the breakdown by ARIA classification, 80 (30.7%) had intermittent rhinitis, and 180 (69.4%) had persistent rhinitis. For both types, the most frequently reported severity was “moderate” (67.7% vs 20.8% for “mild” and 11.6% for “severe”). The most frequently reported concomitant diseases were conjunctivitis (52/161 [32%] in those with HDM allergy and 68/99 [69%] in patients with grass pollen allergy; P<0.05) and asthma (89/161 [55%] for HDM allergy and 44/99 [45%] for grass pollen allergy; not significant). The only other concomitant disease reported in more than 10% of the population was atopic dermatitis. Of the 133 patients with asthma, 53% had intermittent disease, 31.6% mild persistent disease, and 15.4% moderate persistent disease according to the GINA classification, while 59.8% of those with asthma had controlled disease, 21.4% had partially controlled disease and 2.6% had uncontrolled disease. No data on asthma control were available in 16.2% of patients.
Disease characteristics of the paediatric population analysed.
| Overall population (N=1043) | Children (N=260) | P valuea | |
| Change in symptoms since diagnosis | 0.188 | ||
| Much worse | 72 (7.0%) | 15 (5.9%) | |
| Worse | 466 (45.2%) | 106 (40.6%) | |
| No change | 323 (31.4%) | 102 (39.1%) | |
| Better | 149 (14.5%) | 35 (13.3%) | |
| Much better | 20 (1.9%) | 3 (1.1%) | |
| ARIA classificationb | 0.159 | ||
| Intermittent | 271 (26.4%) | 80 (30.7%) | |
| Mild | 27 (10.4%) | ||
| Moderate | 50 (19.1%) | ||
| Severe | 3 (1.2%) | ||
| Persistent | 755 (73.6%) | 180 (69.4%) | |
| Mild | 27 (10.4%) | ||
| Moderate | 126 (48.6%) | ||
| Severe | 27 (10.4%) | ||
The most frequently used diagnostic test was visual inspection (89% patients with HDM allergy and 83% with grass pollen allergy). Auscultation was also common (78% for HDM allergy and 77% for grass pollen allergy). Rhinoscopy, the next most commonly reported diagnostic test, was used significantly more often in patients with HDM allergy (74%) than those with pollen allergy (59%) (P<0.05). Likewise, palpation was used significantly more often in patients with HDM allergy (32% vs 11%; P<0.05).
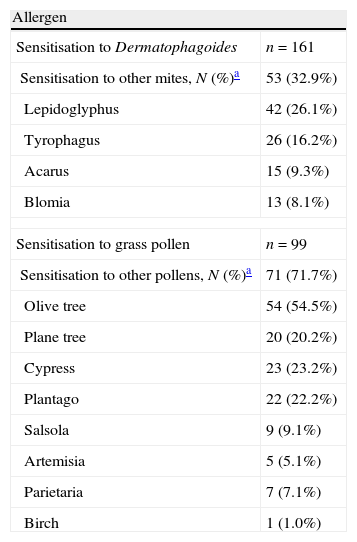
In the skin-prick tests, 37% of the patients allergic to HDM were sensitised exclusively to Dermatophagoides, whereas only 17% of those with grass pollen allergy were sensitised exclusively to grass pollen. Table 3 summarises sensitisation to other HDMs or other pollens. The other pollen to which patients with grass pollen sensitisation were most frequently sensitised was olive tree pollen (54.5%). Specific IgE was used in 60% with HDM allergy versus 47% with grass pollen allergy (P<0.05). Molecular diagnosis (specific IgE with recombinant allergens) was only used in 3% of patients with rhinitis due to grass pollen allergy. Other diagnostic tests performed were spirometry (53% of patients with HDM allergy and 42% with grass pollen allergy). Nasal function tests were only performed in patients with HDM allergy; these included rhinomanometry (in 6% of patients), acoustic rhinometry (in 2%) and nasal peak flow (in 1%).
Sensitisation to house dust mites other than Dermatophagoides and to other pollens other than grass pollens in skin-prick tests.
| Allergen | |
| Sensitisation to Dermatophagoides | n=161 |
| Sensitisation to other mites, N (%)a | 53 (32.9%) |
| Lepidoglyphus | 42 (26.1%) |
| Tyrophagus | 26 (16.2%) |
| Acarus | 15 (9.3%) |
| Blomia | 13 (8.1%) |
| Sensitisation to grass pollen | n=99 |
| Sensitisation to other pollens, N (%)a | 71 (71.7%) |
| Olive tree | 54 (54.5%) |
| Plane tree | 20 (20.2%) |
| Cypress | 23 (23.2%) |
| Plantago | 22 (22.2%) |
| Salsola | 9 (9.1%) |
| Artemisia | 5 (5.1%) |
| Parietaria | 7 (7.1%) |
| Birch | 1 (1.0%) |
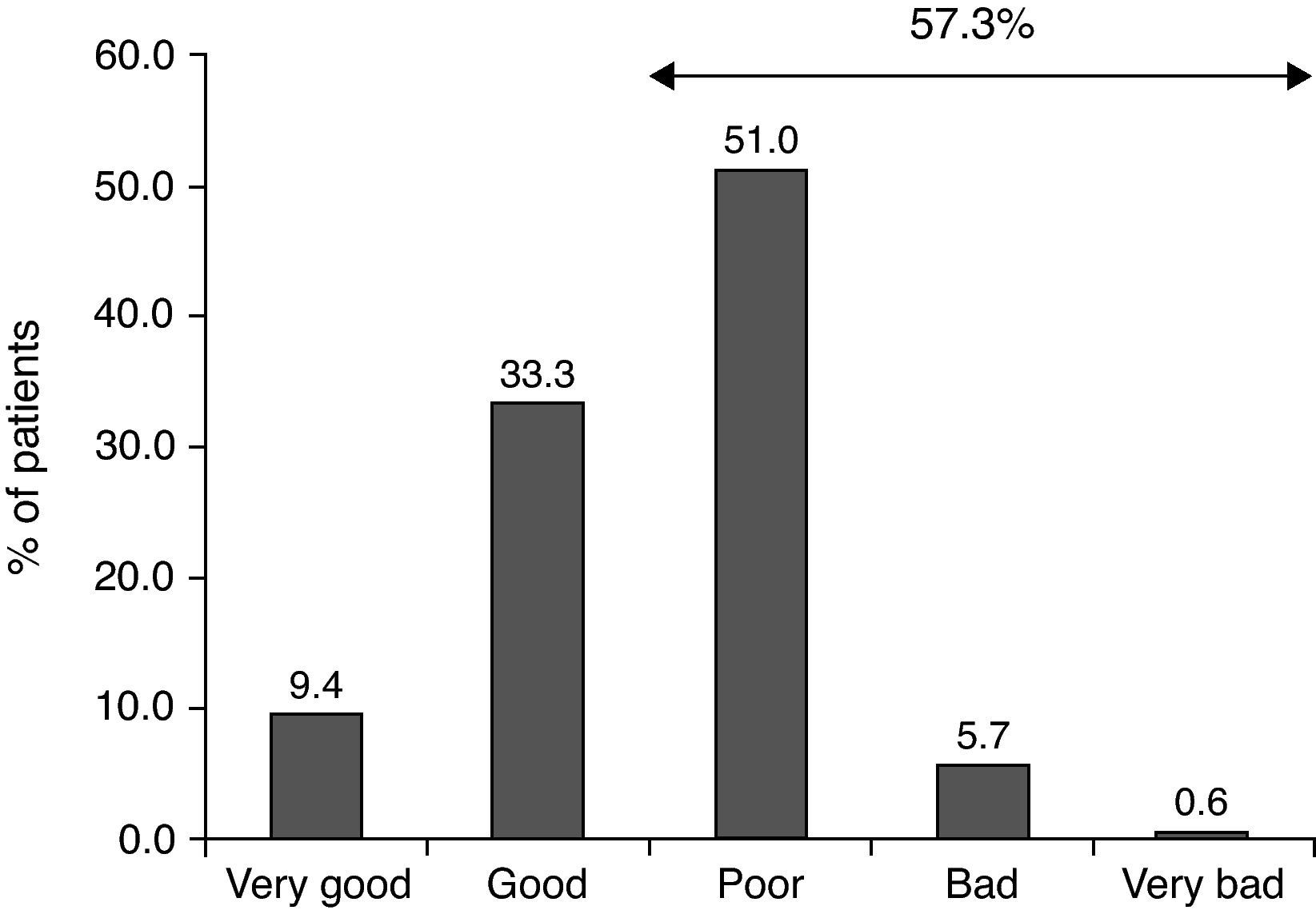
The most common therapeutic measures were allergen avoidance (96.5%), symptomatic treatment (98.5%) and educational measures (94.2%). Control of disease with symptomatic treatments was reported, in a retrospective way, as poor, bad or very bad in 57.3% of the patients (Fig. 1).
Allergen specific immunotherapy was administered in 56.9% of the patients. Of patients with HDM allergy who received specific immunotherapy (62.7% of all patients with HDM allergy), the route of administration was subcutaneous (allergoid or depot) in approximately half while the other half received sublingual specific immunotherapy. In the case of patients with grass pollen allergy, 48.0% received immunotherapy, and the sublingual route was preferred (59.6% of the courses of specific immunotherapy administered). With regard to the composition of the immunotherapy courses administered, for patients with grass pollen allergy, 39% also included olive tree pollen while for patients with HDM allergy only 8% included a mixture of Dermatophagoides and other mite species.
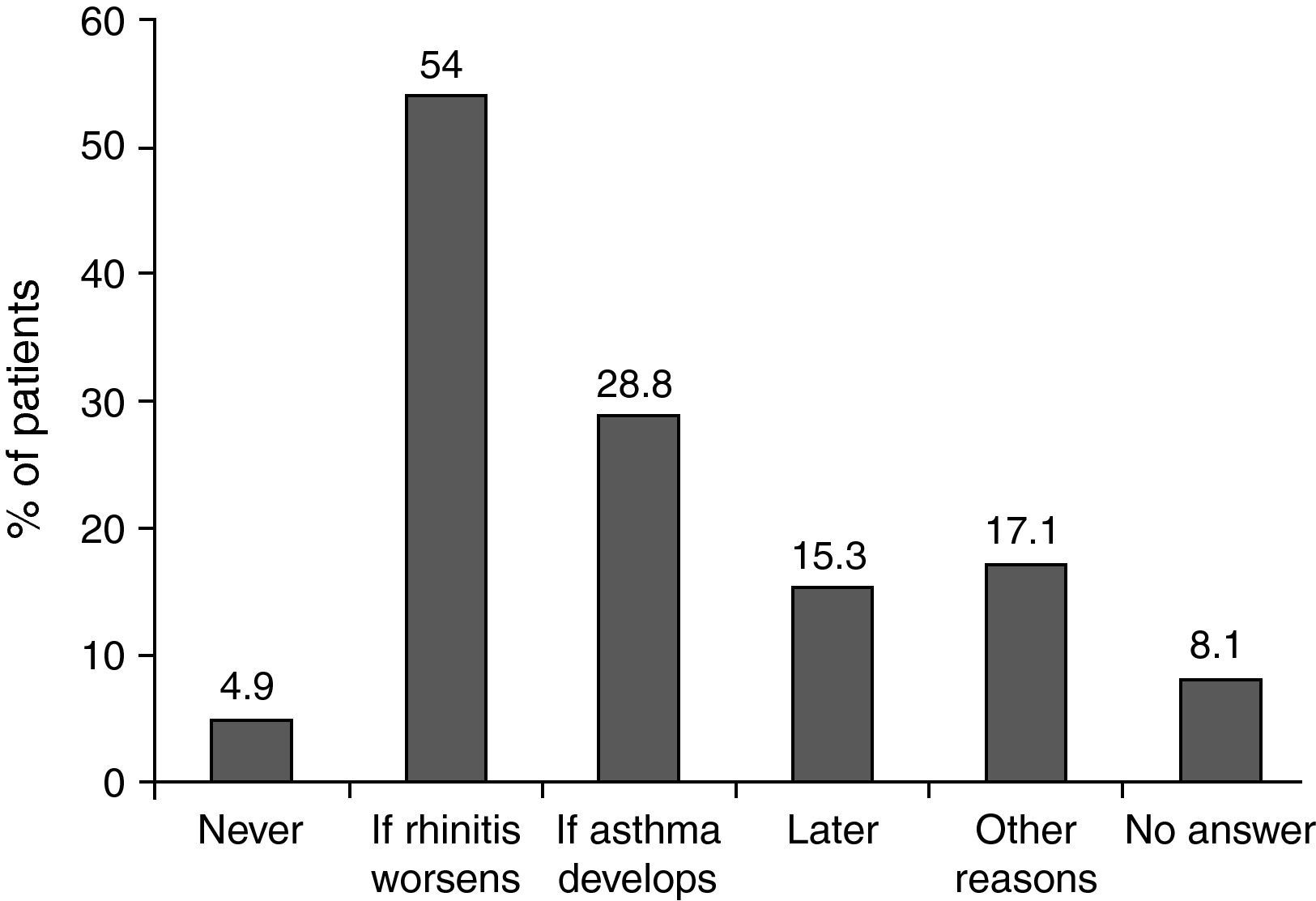
When investigators were asked why they did not prescribe specific immunotherapy in certain patients, two thirds replied that they preferred to observe the course of the disease (54% would prescribe immunotherapy if the disease worsened and 28.8% if the patient developed asthma [Fig. 2]). These patients mostly had moderate intermittent or persistent disease (20.6% and 45.6% of patients with a wait-and-see approach) and no change (47.0%) or a worsening (32.4%) of symptoms since onset of the disease. Concomitant asthma was reported in 39.1%.
DiscussionMost of the paediatric population included in our study had moderate or severe rhinitis, with a disease duration of approximately four years. Although symptoms are thought to improve as children become adults,18 little or no improvement was observed for the ages covered by our study. Moreover, control of disease with symptomatic treatments was generally considered inadequate. Such treatments need to be considered carefully in children.19 Some of the drugs available to treat allergic rhinitis do not have full paediatric approval and the way that a drug is distributed and metabolised may differ greatly between children and adults. In addition, there has been some debate about whether corticosteroids hinder growth20 and the fear of adverse events makes them unpopular with patients.
In view of the concerns about symptomatic treatment, and given the poor control achieved, it is surprising that many specialists opted for a “wait-and-see” approach before prescribing specific immunotherapy. In general, the patients in whom a “wait-and-see” approach was applied had mild or intermittent disease, although substantial proportions had moderate intermittent or persistent disease (20.6% and 45.6%, respectively) and nearly one-third had experienced worsening symptoms since the onset of the disease. This wait-and-see approach could be questioned, given that the potential benefits of specific immunotherapy extend beyond an improved control of symptoms of allergic rhinitis. For example, some studies suggest that it can help prevent the development of polysensitisation21 as well as asthma22,23 later in life.
Educational measures were applied in almost 95% of the population. However, of the patients receiving symptomatic treatments, only 58% received specific educational measures about their treatment, perhaps assuming that the patients or their parents would read the patient information leaflet. Nevertheless, in view of the relatively poor control of disease with symptomatic agents, this is surprising. Greater effort in explaining the symptomatic treatment to the patients or their parents might improve adherence and therefore control of the disease. In the case of patients receiving specific immunotherapy, approximately one-fifth did not receive any educational intervention and only one-third of the patients were given a follow-up diary. Such measures might be useful to ensure that patients keep their appointments and are more compliant.
It is important to have an accurate diagnosis of the allergens implicated as this will affect any avoidance advice offered and is crucial when specific immunotherapy is prescribed. According to the skin-prick tests, poly-sensitisation was the rule: 37% of the patients with HDM allergy were sensitised exclusively to Dermatophagoides and only 17% of those with grass pollen allergy were sensitised exclusively to grass pollen. These differences in mono- and poly-sensitisation were reflected in the allergens included in the specific immunotherapy, with 39% of immunotherapy courses for grass pollen allergy also including olive tree pollen. Of note was the limited use of molecular diagnosis (specific IgE with recombinant allergens) for a more accurate diagnosis in grass-pollen allergic patients co-sensitised to olive tree pollen. Nasal function tests were not widely used, although such tests could provide very valuable information in clinical practice.
Asthma was frequently reported as a concomitant disease (55% in patients with HDM allergy, and 45% in patients with grass pollen allergy). The greater prevalence of asthma in patients with HDM allergy is in line with the findings of other studies.24 In addition, poly-sensitised allergic rhinitis patients have also been reported to be at a greater risk of having concurrent asthma,25 although our analysis did not provide any information in this regard. Spirometry was performed in about 50% of patients. Nevertheless, given this greater risk for asthma, spirometry should be performed in all patients regardless of whether or not they have asthma, as advised by some guidelines.12
In conclusion, in this observational study of paediatric patients with allergic rhinitis attending specialists, patients tended to have moderate-severe allergic rhinitis. For many patients, symptoms were either not improving or worsening and more than half did not improve with symptomatic treatments, although these were extensively prescribed. In the case of immunotherapy, many specialists preferred a wait-and-see approach. Asthma was a common concurrent disease in these patients, in agreement with the widely accepted view that these two diseases, one of the upper airway and the other of the lower airway, are often associated.
Conflict of interestJ.L. Justicia is an employee of Stallergenes Ibérica, S.A.
FundingThis study was funded by Stallergenes Ibérica S.A.
We thank Content Ed Net Madrid for editorial assistance.
PETRA Study: Proyecto Epidemiológico y observacional relacionado con el abordaje Terapéutico de la Rinitis Alérgica.