The use of insect repellent has increased during recent years to prevent insect stings and its feared consequences of virus and parasitic diseases transmitted by infected mosquitoes and tick bites. DEET (N, N-diethyl-3-toluamide) is the most effective and most widely-used insect repellent and it is used by approximately 30% of the population.1,2
The DEET Registry is a post-marketing surveillance system which has collected voluntary reports of different and infrequent moderate to severe adverse events to this drug, including seizures and other neurological symptoms, dermal rashes and other systemic manifestations. Hives, rashes, itching, redness and swelling after exposure were detected in 85 (35%) out of 242 cases.2 Hypersensitivity reactions manifested as contact urticaria and anaphylaxis, after brief contact with DEET, have also been described as case reports.3–6 In some patients with hypersensitivity to the product, skin test with DEET induced a typical immediate wheal and flare reaction.3,5 Furthermore, passive human sensitisation was reported positive, suggesting an IgE mediated phenomena.3,5 However, a skin test negative patient has also been reported.7
Contact urticaria has been described for a number of stimuli. Allergens from shrimp or latex react through an IgE mediated reaction, while others, like water induced urticaria or contrast media reactions, are unlikely to represent an IgE mediated response.8 Flow cytometry detection of basophile activation has been utilised previously for the diagnosis of allergy.9 Basophile activation test (BAT) takes advantage of CD63 over expression by activated basophiles. It has been described as a useful tool to explore the capacity of a substance to mediate cell activation and to suggest the possible mechanistic way of cell degranulation. Furthermore, for a number of allergens, IgE induced-basophile degranulation has been well documented. However, for pseudo-allergens, like non-steroidal anti-inflammatory drugs, basophile activation does not seem to increase CD63 or other cell membrane markers expression. So, BAT response of basophiles from a sensitive patient to DEET may contribute to our understanding of the physiology of DEET hypersensitivity reaction.
A 50-year-old woman consulted with a history of an urticarial reaction to DEET-based insect repellents. She stated that a few minutes after either spray, aerosol or lotion product application, an urticarial rash appeared on the exposed areas. She tried several different brands of repellent with the same result. Since she frequents a mosquito-infested zone she decided to consult. An open-label challenge was performed spraying a DEET containing product on her right antecubital fossae. In a few minutes, an urticarial and pruriginous rash developed which lasted for 60min.
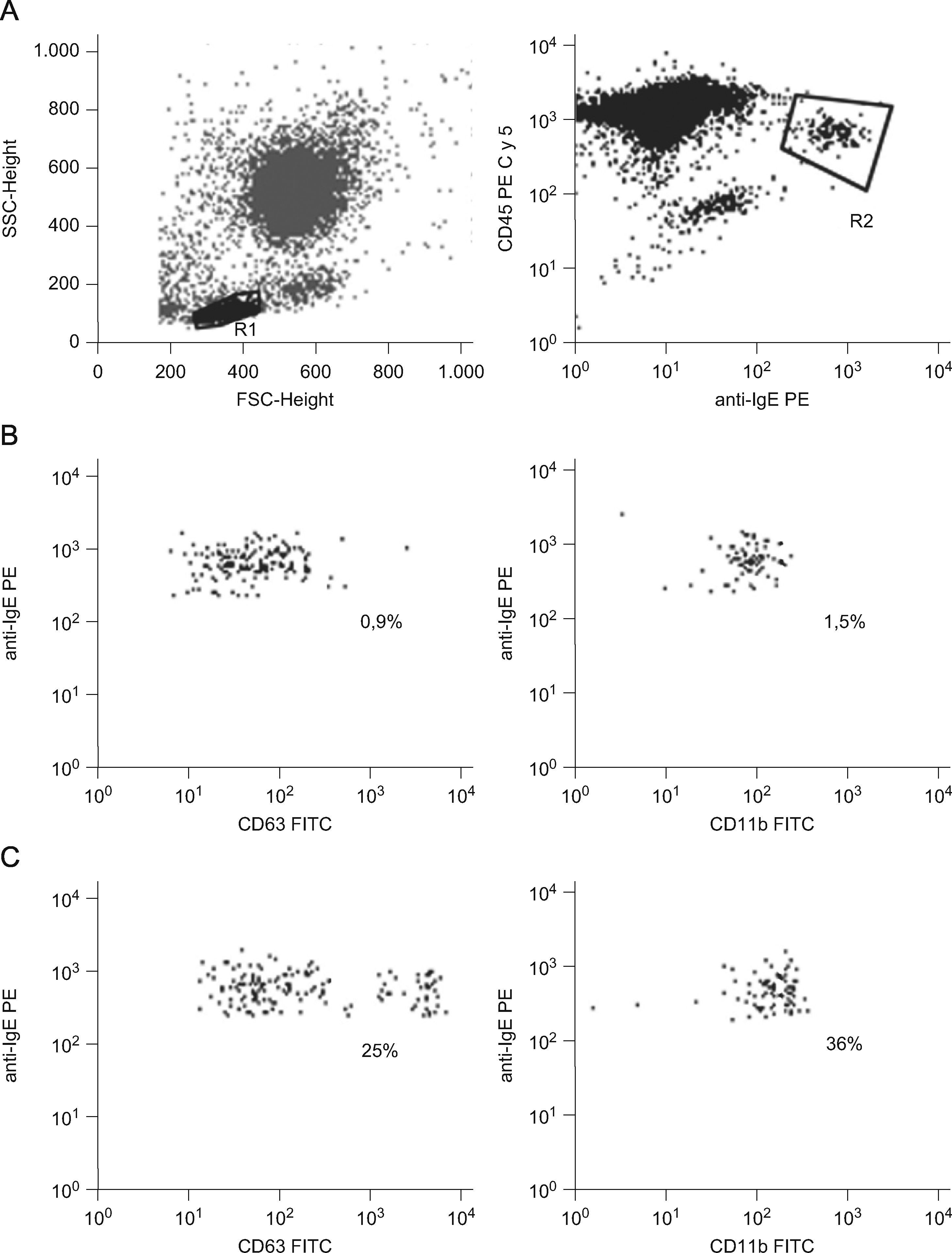
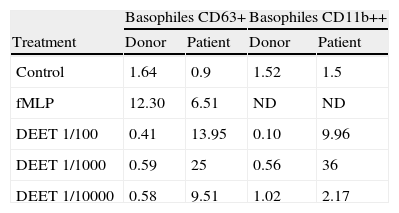
We evaluated the CD63 and CD11b expression on basophiles from the patient after incubation of 100μl of peripheral blood with DEET (1/100, 1/1000 or 1/10000) during 30min at 37°C. Cells in PBS (Phosphate Buffer Saline) and stimulated with fMLP (formyl-metionyl-leucil phenilalanina) were used, respectively, as negative and positive controls. Blood from a DEET-tolerant healthy donor was studied simultaneously. After incubation, cells were labelled with monoclonal antibodies anti-CD63 FITC (clone H5C6) or anti-CD11b FITC (clone Bear1), anti-CD45 PE-Cy5 (clone IMMU19.2) and biotinylated polyclonal anti-human IgE made in goat (Vector) followed by PE streptavidin and analysed with a FACScan flow cytometer (Becton Dickinson). Figure 1 displays dot plots showing the isolated basophiles according to their forward (FSC) and side (SSC) scatter characteristics (R1) and anti-IgE/anti-CD45 fluorescence (R2). The percentages of activated basophiles (IgE++CD63+ or IgE++CD11b++) were recorded (Table 1). The drug clearly activated patient’s basophiles demonstrating specific DEET hypersensitivity.
Flow Cytometry analysis of basophiles from the DEET sensitive patient stimulated with the drug. A: Basophiles were identified in R1 according to FSC-SSC characteristics and their staining with anti-IgE/anti-CD45 fluorescence (R2). B and C: Basophiles expressing CD63 and CD11b in the negative control (B) and after DEET stimulation (C). Percentages are indicated.
BAT results: Percentages of basophiles expressing CD63 or CD11b in the DEET sensitive patient and a tolerant donor.
| Basophiles CD63+ | Basophiles CD11b++ | |||
| Treatment | Donor | Patient | Donor | Patient |
| Control | 1.64 | 0.9 | 1.52 | 1.5 |
| fMLP | 12.30 | 6.51 | ND | ND |
| DEET 1/100 | 0.41 | 13.95 | 0.10 | 9.96 |
| DEET 1/1000 | 0.59 | 25 | 0.56 | 36 |
| DEET 1/10000 | 0.58 | 9.51 | 1.02 | 2.17 |
Basophiles from peripheral blood were incubated with Buffer (Control), different dilutions of DEET or fMLP (Positive control). Cells were stained with anti-CD63 or anti-CD11b/anti-IgE/anti-CD45 and analysed by Flow Cytometry.
We also explored DEET interaction with T and B lymphocytes. An in vitro proliferative response to DEET was studied performing 5, 6-carboxifluorescein diacetate succinimidyl ester-based (CFSE) proliferative assay as described.10 Cells were stimulated with 1/100, 1/1000 and 1/5000 DEET dilutions and without the drug as negative control. Stimulation with Tetanus Toxoid (TT) was used as positive control. After that, cells were aliquoted in two equal volumes and labelled with anti-CD4 PE (clone SK3)/anti-CD3 PECy5 (clone UCHT1) for T lymphocytes, and anti-CD19 PECy5 (clone HIB19) for B lymphocytes respectively. The percentages of DEET-reactive lymphocytes were recorded as those with lower CFSE fluorescence intensity as compared to non-stimulated cells, showing a homogenous bright CFSE label. TT induced 7% of proliferation compared to 1% in the negative control. Patient’s PBMC proliferative responses to DEET were negative with all doses and similar to those of a DEET tolerant donor.
We conclude that DEET hypersensitivity in our patient is an IgE mediated response and that the intimate physiology of the reaction takes place inducing mast cell and basophile degranulation in a way which increases CD63 expression. T and B cell proliferative responses did not suggest direct DEET lymphocyte activation.