Cutaneous eruptions emerging shortly after haematopoietic stem cell transplantation (HSCT) pose a particular diagnostic challenge for oncologists and consulting dermatologists. Many distinct dermatologic entities occur early in the post-HSCT period, including acute graft-versus-host disease (GVHD), viral exanthema, eruption of lymphocyte recovery, erythema multiforme, toxic erythema of chemotherapy and morbilliform drug eruptions.1–3 These diseases are often clinically indistinguishable, and their similar histopathological features may preclude a definitive diagnosis, leading to unfavourable patient outcomes.
Exposure to medications that were previously allergenic to either the stem cell donor or recipient may have implications for the post-transplant course of the HSCT patient.3 The interplay between donor and recipient drug allergy, and its influence on HSCT outcomes has yet to be studied in a systematic manner. It has been suggested that allogeneic HSCT patients can be safely exposed to medications to which they were previously allergic,4 but there are no reports in the literature addressing the consequences of post-HSCT patient exposure to drugs that caused an allergic response in donors. Furthermore, the potential relationship between drug allergy and other cutaneous complications in HSCT patients, such as GVHD, has not been examined.
Our goal was to determine how many post-HSCT patients received medications that they or their donors were allergic to, and characterise any ensuing reactions (cutaneous or otherwise) that occurred. We additionally hypothesized the incidence of acute GVHD to be greater in HSCT recipients that received these medications post-transplant, assuming drug allergy as a contributor to immune activation.
A retrospective chart review was conducted to identify all first-time adult allogeneic HSCT recipients in the Northwestern Memorial Hospital system in 2009. Information collected included demographic and disease-specific information, as well as documented allergies of both stem cell donors and recipients. Medication exposures during the first 60 days post-transplant were recorded and cross-referenced with drug allergies. Incidence and diagnosis of all skin eruptions during this time were also recorded, including acute GVHD.
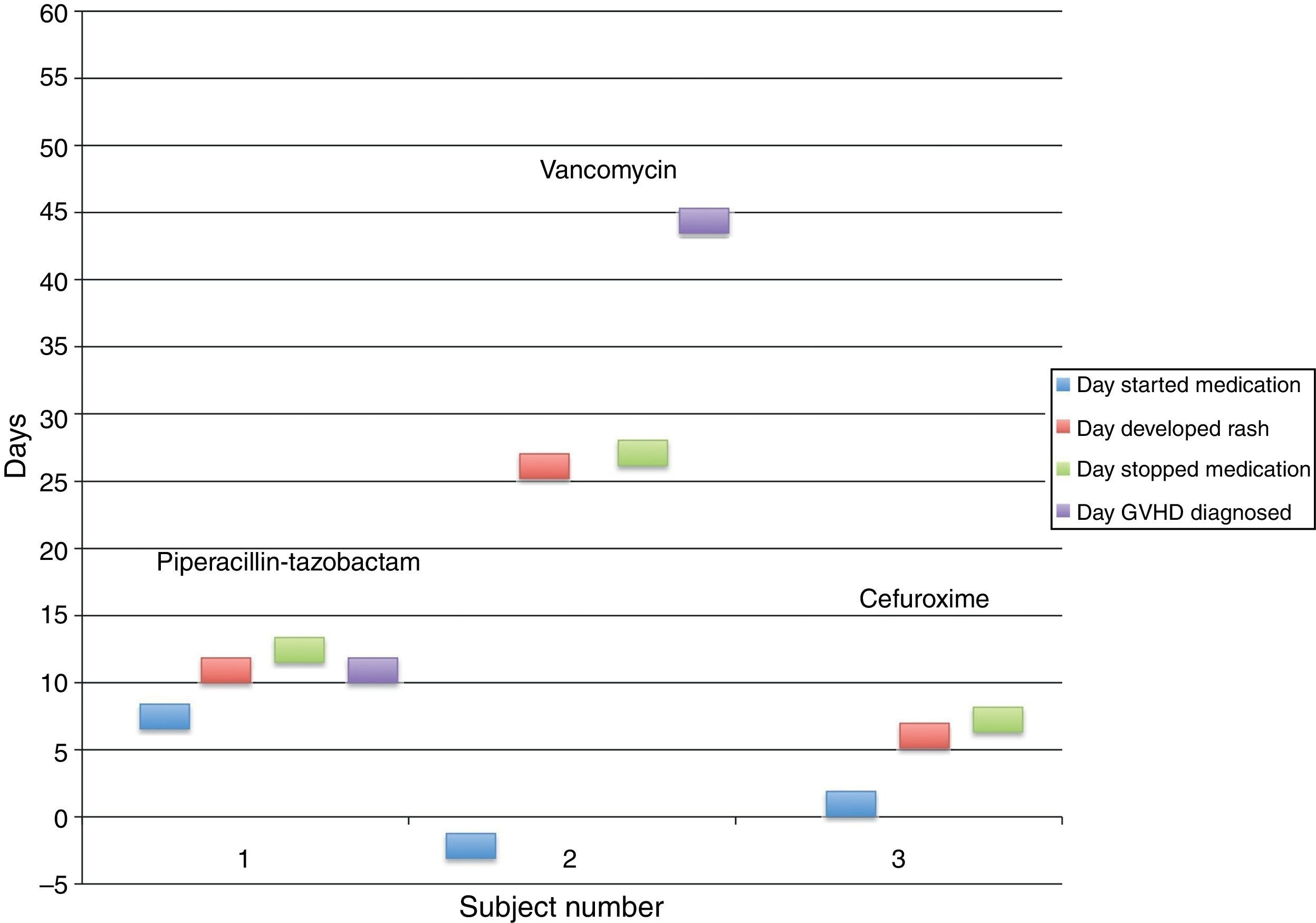
We found 59 first-time adult allogeneic HSCT patients in 2009. Two patients were exposed to previously documented allergenic medications (subject 1: piperacillin–tazobactam; subject 2: vancomycin), and both patients developed acute GVHD with cutaneous involvement (Table 1). One additional patient (subject 3) received cefaclor post-HSCT without subsequent development of GVHD, despite the fact that the stem cell donor was allergic to cefuroxime, also a cephalosporin (Fig. 1).
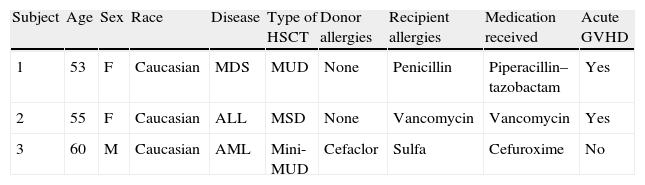
Characteristics of subjects exposed to medications with prior hypersensitivity.
| Subject | Age | Sex | Race | Disease | Type of HSCT | Donor allergies | Recipient allergies | Medication received | Acute GVHD |
| 1 | 53 | F | Caucasian | MDS | MUD | None | Penicillin | Piperacillin–tazobactam | Yes |
| 2 | 55 | F | Caucasian | ALL | MSD | None | Vancomycin | Vancomycin | Yes |
| 3 | 60 | M | Caucasian | AML | Mini-MUD | Cefaclor | Sulfa | Cefuroxime | No |
MUD, matched unrelated donor Mini; MUD, non-myeloablative matched unrelated donor; MSD, matched sibling donor; MDS, myelodysplastic syndrome; ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia.
Subject 1 developed a morbilliform eruption on the arms on post-HSCT day +14, four days after starting piperacillin–tazobactam. Diarrhoea was reported concurrently with the skin eruption, while serum total bilirubin remained within normal limits. While the patient was diagnosed clinically with acute GVHD of the skin and gut, the dermatology service was not consulted, and no skin biopsy was performed.
Subject 2 developed an erythematous and petechial eruption on the dorsal and plantar feet on post-HSCT day +30, 31 days after starting vancomycin. Skin biopsy was consistent with grade II GVHD, and no diarrhoea or abnormal liver function tests were present.
Subject 3 was exposed to cefuroxime on post-HSCT day +2; the donor had a documented allergy to cefaclor, another second-generation cephalosporin with similar chemical structure. The patient subsequently developed an episode of “hives” on the left chest and jaw five days later, accompanied by mild diarrhoea and an increased serum total bilirubin of 1.7mg/dL. Dermatology did not evaluate the patient and GVHD was never diagnosed. Cefuroxime was discontinued without any subsequent cutaneous events.
We observed the development of acute GVHD in 2/2 patients exposed to prior allergenic medications following myeloablative allogeneic HSCT. An urticarial reaction was also observed in a non-myeloablative allogeneic HSCT recipient after receipt of a structurally similar donor-allergenic medication; though we cannot entirely exclude the possibility that this urticarial reaction did not represent acute GVHD, as no dermatologic consultation or skin biopsy was provided. The context for prescribing these medications in all three cases (risk-benefit analysis versus clerical oversight) is unknown. Under ideal circumstances, medications administered post-HSCT would exclude agents to which the recipient or donor previously demonstrated a hypersensitivity reaction.
There are limitations to the conclusions drawn from these observations, most notably the small sample size and the high incidence of acute GVHD following allogeneic HSCT. Additionally, this was a retrospective review and only one of the three subjects was evaluated by a consulting dermatologist. While oncologists and other health care providers may routinely evaluate skin eruptions in HSCT patients, clinical assessment by an experienced dermatologist remains the standard of care at most centres.
Transfer of atopy and contact allergy from donor to recipient following bone marrow transplantation has been described,5,6 and the presumed mechanism is via adoptive transfer of donor memory T cells to a newly reconstituted recipient immune system. There is also evidence of allergen-specific IgE-mediated hypersensitivity passed from donors to recipients of stem cell transplantation via B cell transfer.7 Thus, HSCT may predispose certain patients to the development of atopy or new environmental and medication allergies.
Whether drug allergies persist in patients undergoing non-myeloablative HSCT or in HSCT recipients with less than total donor chimerism is unknown, and there are no published data on drug allergy persistence following HSCT.
This observational study suggests that there may be an important immunologic component of drug allergy that may influence the development of acute GVHD. A prospective multi-centred trial with dermatologist evaluation of skin eruptions in HSCT patients would help to further elucidate both the role of medication allergy as a risk factor for GVHD and the presence or absence of drug allergy following transplant.
Ethical disclosuresProtection of human subjectsThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Confidentiality of dataThe authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
Conflict of interestThe authors have no conflict of interest to declare.
This research was supported in part by NIH grants 3R01CA125077-01A1 (D.P.W.) and 3R01CA125077-01A1S1 (D.P.W.). Dr. Cotliar is supported by a career development award from the Dermatology Foundation.