Neonatal jaundice is one of the most common problems that affect newborn infants, and phototherapy is usually used for treatment.
ObjectivesEvaluation of the effect of phototherapy on neonatal immune system through measuring the percentage of B and T lymphocytes and determining the frequency of development of infections and need for hospitalisation during the first six months of life.
MethodsA prospective cohort study was conducted on 50 full term new-borns; 25 with indirect hyperbilirubinaemia and treated with conventional phototherapy and 25 healthy matched neonates as untreated controls. The percentages of CD19+, CD4+ and CD8+ lymphocytes were measured by flow cytometry before phototherapy and 72h after exposure. Follow-up of the study group for the occurrence of infections for a period of six months after phototherapy.
ResultsThe study showed a significant difference in CD19+ lymphocytes percentage between patients before phototherapy and controls (P value<0.01), also a significant correlation between serum levels of total bilirubin in patients and CD19+ lymphocytes percentage (P value<0.05). There was no significant difference between the percentages of CD19+, CD4+ and CD8+ lymphocytes in patients before or after 72h of exposure to phototherapy (P value>0.05). Also, there was no correlation between the percentages of CD19+, CD4+ and CD8+ lymphocytes after 72h of exposure to phototherapy and the occurrence of infections (Gastrointestinal tract and Respiratory tract infection) after six months of follow-up (P value>0.05). More studies are needed with larger number of patients to determine the effect of phototherapy on immune system.
Neonatal jaundice is one of the most common conditions; about 60% of term and 80% of preterm infants develop jaundice in the first week after birth.1 Hyperbilirubinaemia can become dangerous, causing encephalopathy or kernicterus.2 Phototherapy is usually used to treat severe hyperbilirubinaemia in term and large preterm infants.3 During the last few decades, concerns about potential toxic effects of phototherapy have been expressed. One possible harmful consequence is affection of lymphocytes subtypes and subsequently cytokines production which can affect the immune system functions in infants.4 Ultraviolet (UV) light used in phototherapy can cause a number of cellular changes in irradiated skin. Langerhans cells are dendritic antigen presenting cells in the epidermis, upon sensing a danger signal; they migrate to draining lymph nodes where they initiate activation of cell-mediated immunity. UV radiation may damage Langerhans cells and even high doses may lead to their death in the skin.5 Phototherapy could influence the immature immune system of the new-borns, probably by direct effect on T lymphocytes in the thin skin.6,7 UV radiation also causes infiltration of skin by macrophages which then migrate to draining lymph nodes where they produce IL-10 and contribute to the immunosuppressive microenvironment.8 One of the most important outcomes of UV induced suppression of the immune system is impaired activation of effector and memory T lymphocytes as well as increased activation of regulatory T cells.9 UV light, although a small component of neonatal phototherapy, markedly decreases circulating CD4T lymphocyte, interferes with CD8 cytotoxic T lymphocytes, and reduces NKT cell activity. Therefore, it affects the immune system and may lead to development of autoimmunity diseases and allergy.1 The current study was conducted to evaluate the effect of phototherapy on B and T lymphocytes through measuring the percentage of CD19, CD4 and CD8 subsets in new-borns with indirect hyperbilirubinaemia before phototherapy and 72h after exposure and monitoring clinically the frequency of occurrence of infections and need for hospitalisation of these new-borns for a period of six months after phototherapy.
Subjects and methodsThis prospective cohort study was conducted on 50 full term new-borns from neonatal intensive care units in Cairo University paediatric hospitals. Consent was obtained from parents of neonates included in this study.
They were classified into two groups.
1-Patients Group: 25 full term new-borns of gestational age more than 37 weeks, postnatal age less than or equal to 14 days, with neonatal indirect hyperbilirubinaemia and treated by conventional phototherapy based on American Academy of Paediatrics recommendations.10 Phototherapy was carried out as follows: the infants were uncovered except for shielded genitalia and eyes. White fluorescent lamps emitting light at a wavelength of 420–470-nm and placed at 40cm distance from the neonates. All patients were exposed to continuous phototherapy, except during the time of feeding and cleaning.
2-Untreated control group: 25 matched healthy full term new-borns without neonatal jaundice and did not receive phototherapy.
Preterm infants, neonates suffering from sepsis or any congenital anomalies and neonates with hypoxic ischaemic encephalopathy or in need of exchange transfusion were excluded.
Full history taking and clinical examination of the study groups including: birth weight, height, skull circumference and system examination (General, Cardiac, Chest, CNS) was done.
Sample collectionTwo blood samples were collected from neonates with indirect hyperbilirubinaemia: one before phototherapy and the other one 72h after exposure. Only one sample was collected from the untreated control group.
2ml of blood was collected in EDTA vacutainers and analysed by flow cytometry within 24h after collection to measure the percentage of CD19+, CD4+ and CD8+ lymphocytes in peripheral blood.
For flow cytometric analysis the following monoclonal antibodies were used:
- -
Anti-Human CD19 phycoerythrin-cyanine5 (PC5) monoclonal antibody, (Beckman Coulter Company, France, Catalogue Number A07771).
- -
Anti-Human CD4 flourescin isothiocynate (FITC) and CD8 phycoerythrin (PE) monoclonal antibodies Cocktail, (eBioscience Company, Germany, Catalogue Number 22-0408).
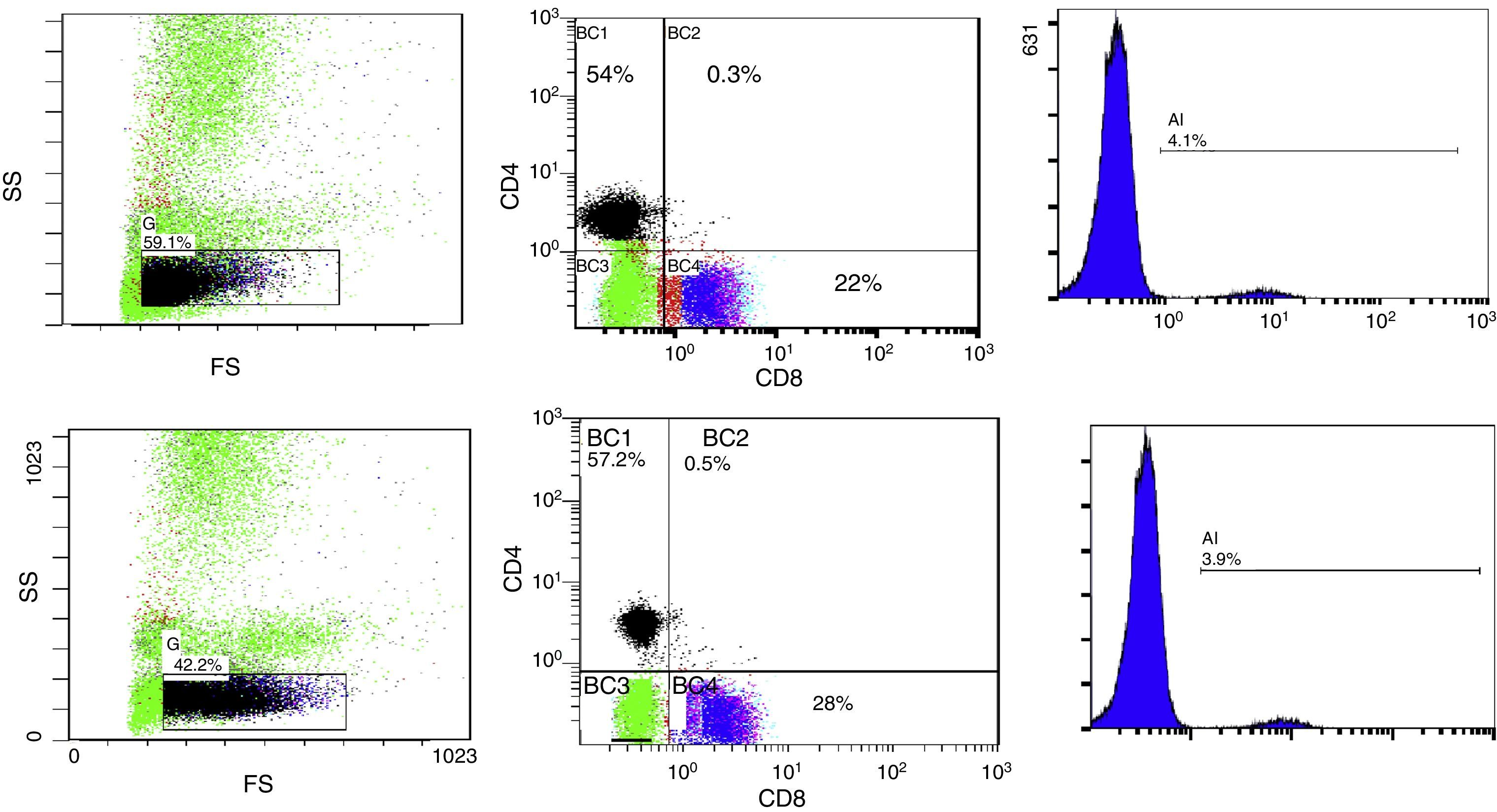
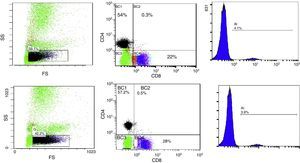
10μl of Anti-Human CD19 PC5 monoclonal antibody and 20μl of Anti-Human CD4 FITC and CD8 PE monoclonal antibodies Cocktail were added to 50μl of the blood sample, followed by incubation for 20min in dark room, then 1ml from lysing reagent was added and finally, incubation for another 20min in dark room. The stained samples were mixed and analysis of lymphocytes was done using EPICS ELITE Coulter flow cytometer (Fig. 1A and B).
Flow cytometric analysis before phototherapy (above) and 72h after exposure (below) showing:
- (1)
The total lymphocyte percentage
- (2)
Percentage of expression of CD4+ (vertical axis) and CD8+ lymphocytes (horizontal axis)
- (3)
Percentage of expression of CD19+ lymphocytes
FS, forward scatter; SS, side scatter; BC, FCM labelling of the region.
Clinical follow-up of patients group for a period of six months after phototherapy for monitoring of occurrence and frequency of infection and need for hospitalisation. Most respiratory virus infections in early childhood are confined to the upper respiratory tract, leading to symptoms of the common cold, with cough, sore throat, hoarseness, runny nose, nasal congestion, headache, low grade fever, and sneezing. Upper respiratory tract infection in infants may lead to lethargy and poor feeding.11 Infective gastroenteritis in young children is characterised by the sudden onset of diarrhoea, with or without vomiting. Most cases are due to a viral infection but some are caused by bacterial or protozoal infections. The illness usually resolves without treatment within days but severe diarrhoea can rapidly cause dehydration.12
Statistical analysisData were collected, tabulated and statistically analysed using SPSS version16.0 as follows: description of qualitative variables as number and percentage: Chi-square test was used to compare qualitative variables between groups, description of quantitative variables as median and range (minimum–maximum): Wilcoxon Signed Ranks test, used to compare two medians and two ranges of the same group before and after treatment. Kruskall Wallis test was used for comparison between more than two medians and two ranges of groups. Mann–Whitney test used to compare between two medians and two ranges of two groups. The level of significance: P>0.05 means no significance, P<0.05 means significant and P<0.01 means highly significant.
Ethical considerationsApproval of the study protocol was obtained from the Ethical Committee at the Faculty of Medicine, Cairo University. Informed consent was obtained directly from the legal guardian of each patient before data collection and after explanation of the study objectives.
ResultsThe study was conducted on 50 full term new-borns; 25 with indirect hyperbilirubinaemia, 15 males (60%) and 10 females (40%) and 25 healthy matched neonates as untreated control group, 14 males (56%) and 11 females (44%). There was no significant difference among studied groups as regard mode of delivery; 12 (48%) of patients were normal vaginal delivery and 13 (52%) were caesarean section as compared to the controls where 13 (52%) were normal vaginal delivery and 12 (48%) were caesarean section with P value (0.777). There was no significant difference between patients 38 (37–40) and controls 38 (37–41) regarding gestational age (weeks) with P-value (0.416). Also, there was no significant difference between patients 3.2 (2.5–3.9) and controls 3.1 (2.6–3.6) concerning the weight (kg), P-value (0.445).
CBC of the patients before phototherapy and controls were within the normal range for their age: Haemoglobin (11.5–15.6g/dl) in patients and (12.5–15.1g/dl) in controls; white blood cells (WBCs) (6–14×103/μl) in patients and (7–13×103/μl) in controls; lymphocytic count (LC) (3–6.1×103/μl) in patients and (3.3–7.5×103/μl) in controls; and platelets count (160–365×103/μl) in patients and (215–325×103/μl) in controls.
Before phototherapy, there was a significant difference between the median value of WBCs in patients (11.05) and controls (8.4) with P-value (0.013) and a highly significant difference between the median value of LC (4.2) in patients and (5.0) in controls with P-value (0.005). After 72h of exposure to phototherapy, there was no significant difference in WBCs between patients before (11.05) and after (10.5) with P-value (0.205), also between LC before (4.2) and after (4.6) with P-value (0.169).
There was a highly significant difference in levels of total serum bilirubin (TSB) among patients before phototherapy 19.5 (14.8–24.2) and untreated control group 1.8 (1.1–2.9) (P value<0.01) and between patients before 19.5 (14.8–24.2) and after phototherapy 8.9 (6.5–11) (P value<0.01). Phototherapy reduced TSB by more than 50%. 72h was the average duration needed for these patients to end their treatment by phototherapy and adequate to monitor changes that could affect the B and T lymphocytes.
- (1)
Statistical analysis of the percentage of lymphocytes and CD19, CD4 and CD8 expression on lymphocytes by flowcytometry among the studied groups:
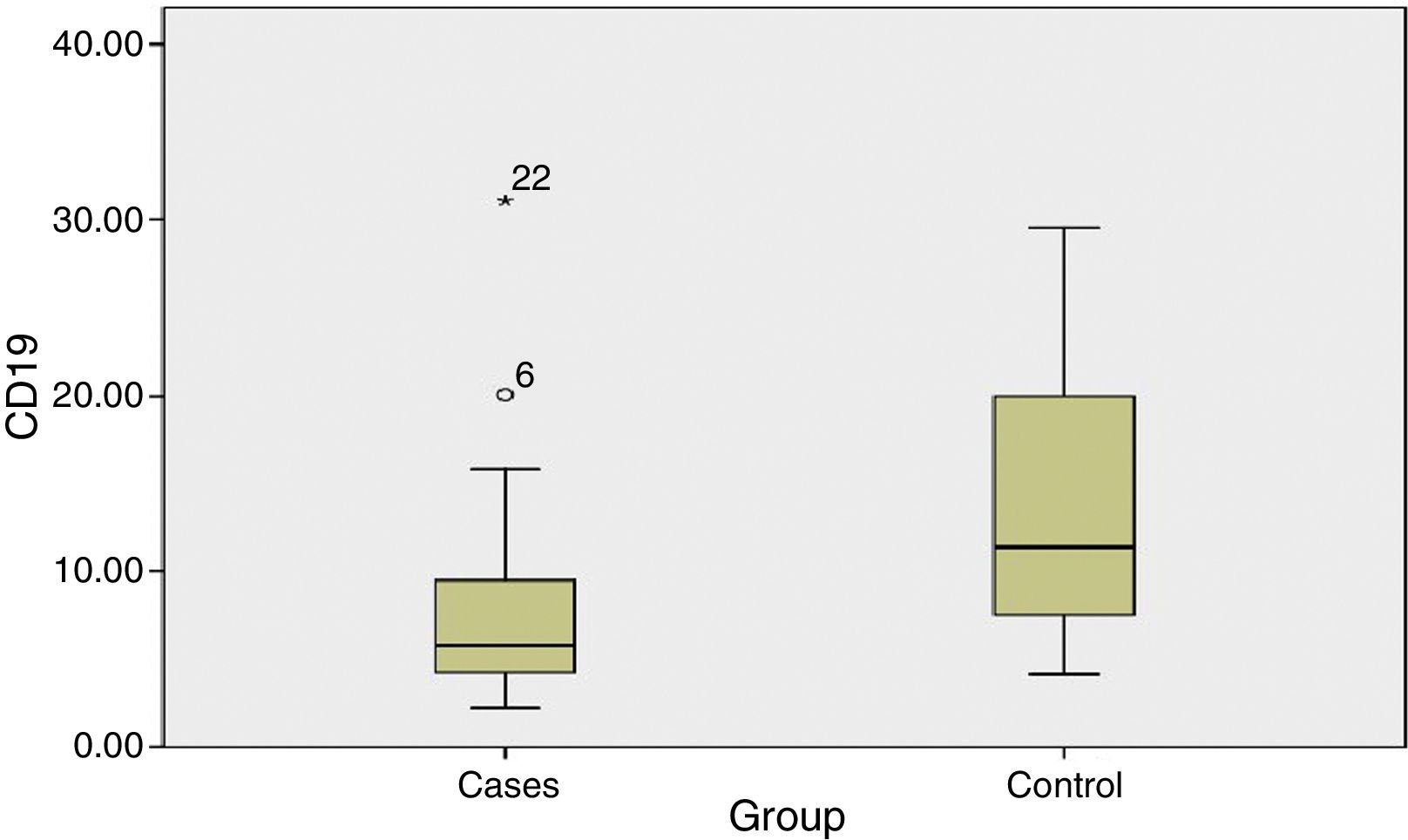
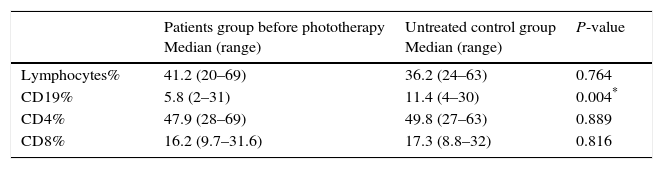
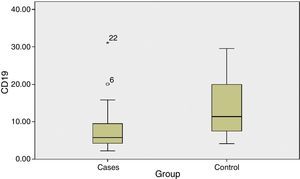
The percentage of CD19+ lymphocytes was higher in the untreated control group as compared to patients before phototherapy with a highly significant difference (P value 0.004).
There was no significant difference in the percentage of lymphocytes, CD4 and CD8 among patients before phototherapy and untreated control group (P value >0.05) (Table 1) (Fig. 2).
Table 1.Statistical comparison between patients and untreated control group as regards Lymphocytes, CD19, CD4 and CD8%.
Patients group before phototherapy
Median (range)Untreated control group
Median (range)P-value Lymphocytes% 41.2 (20–69) 36.2 (24–63) 0.764 CD19% 5.8 (2–31) 11.4 (4–30) 0.004* CD4% 47.9 (28–69) 49.8 (27–63) 0.889 CD8% 16.2 (9.7–31.6) 17.3 (8.8–32) 0.816 - (2)
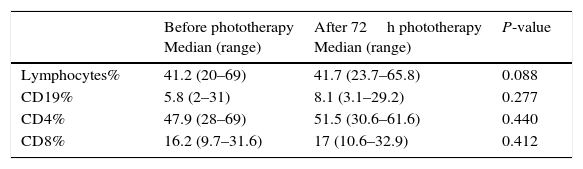
Statistical analysis of the percentage of lymphocytes and CD19, CD4 and CD8 expression on lymphocytes by flowcytometry in patients before and after phototherapy:
There was no significant difference in the percentage of lymphocytes, CD19, CD4 and CD8 between patients before and after 72h of exposure to phototherapy (P value>0.05) (Table 2).
Table 2.Statistical comparison between patients before and after phototherapy as regards Lymphocytes, CD19, CD4 and CD8%.
Before phototherapy
Median (range)After 72h phototherapy
Median (range)P-value Lymphocytes% 41.2 (20–69) 41.7 (23.7–65.8) 0.088 CD19% 5.8 (2–31) 8.1 (3.1–29.2) 0.277 CD4% 47.9 (28–69) 51.5 (30.6–61.6) 0.440 CD8% 16.2 (9.7–31.6) 17 (10.6–32.9) 0.412 - (3)
CD4/CD8 ratio among studied groups and patients before and after 72h of phototherapy:
Statistical comparison of CD4/CD8 ratio between patients before phototherapy 2.8 (1.53–5.31) and the untreated control group 3.02 (1.12–6.11) and patients before 2.8 (1.53–5.31) and after 72h of phototherapy 2.8 (1.2–4.73) showed no significant difference (P value>0.05).
- (4)
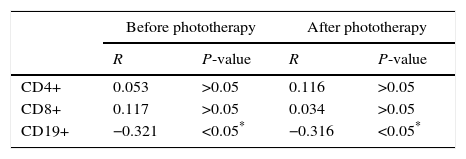
Correlation between serum level of total bilirubin (TSB) and percentages of CD4+, CD8+ and CD19+ lymphocytes among patients (before and after phototherapy):
There was a significant correlation between level of TSB and CD19+ lymphocytes percentage in patients before (P-value<0.05) and after phototherapy (P-value<0.05), but there was no correlation between level of TSB and lymphocytes, CD4+ and CD8+ lymphocytes percentage (P-value>0.05) (Table 3).
- (5)
Follow-up of patients after six months:
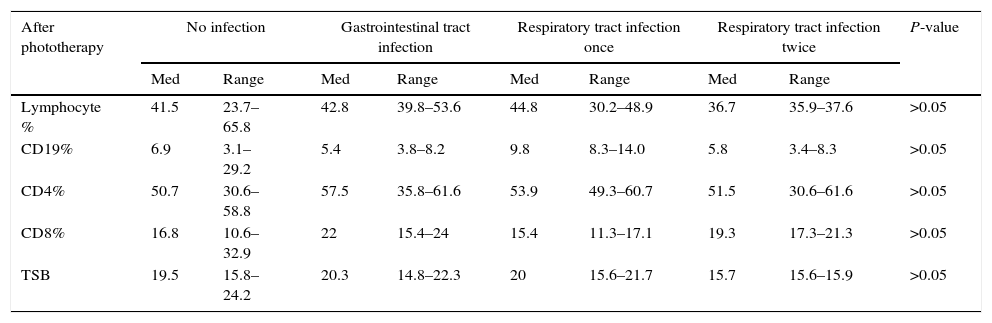
Correlations between levels of lymphocytes, CD19 CD4 and CD8 percentage after phototherapy and infection in patients for six months:
There was no significant correlation between levels of lymphocytes, CD19, CD4 and CD8% and TBS in patients after phototherapy and clinical follow-up for occurrence and frequency of infection for a period of six months after admission (Table 4).
Table 4.Correlations between patients’ laboratory results and clinical follow up.
After phototherapy No infection Gastrointestinal tract infection Respiratory tract infection once Respiratory tract infection twice P-value Med Range Med Range Med Range Med Range Lymphocyte % 41.5 23.7–65.8 42.8 39.8–53.6 44.8 30.2–48.9 36.7 35.9–37.6 >0.05 CD19% 6.9 3.1–29.2 5.4 3.8–8.2 9.8 8.3–14.0 5.8 3.4–8.3 >0.05 CD4% 50.7 30.6–58.8 57.5 35.8–61.6 53.9 49.3–60.7 51.5 30.6–61.6 >0.05 CD8% 16.8 10.6–32.9 22 15.4–24 15.4 11.3–17.1 19.3 17.3–21.3 >0.05 TSB 19.5 15.8–24.2 20.3 14.8–22.3 20 15.6–21.7 15.7 15.6–15.9 >0.05 In conclusion, our study showed that exposure to phototherapy has no direct effect on B and T lymphocytes of the new-born, nor on the occurrence of infections during a period of six months of follow up. However, phototherapy decreased the level of TBS which led to increase in the percentage of CD19+ lymphocytes in patients after 72h of exposure and reached a percentage close to that in the untreated control group.
Phototherapy, a non-invasive intervention, has been used for the treatment of neonatal hyperbilirubinaemia for more than half a century. It is an effective method at wave lengths 425–475nm for treating neonatal jaundice by reducing the plasma concentration of unconjugated bilirubin and hence to prevent kernicterus.1,13
Studies demonstrated the ability of unconjugated bilirubin to cause immunotoxicity besides neurotoxicity.14 Both intensive and conventional phototherapy cause endogenous mononuclear lymphocytes DNA damage and this appeared to be in relation to the duration of exposure, as the damage increased significantly with the duration of phototherapy, as shown by measurements at 24, 48 and 72h.15–17 Lymphocytes are the key mediators of adaptive immune system. Two major types of these lymphocytes are B and T lymphocytes which mediate humoral and cell mediated immunity respectively.18
In our study, although CD19+ lymphocytes percentage was significantly higher in the untreated control group (11.4) as compared to patients before phototherapy (5.8) (P value 0.004), there was no significant difference in CD19 between patients before phototherapy (5.8) and after 72h (8.1) (P value 0.277). But still, there was around 40% increase in the percentage of CD19+ lymphocytes 72h after phototherapy which would be closer to the untreated control group (11.4) (P-value 0.801). This increase was highly related to the level of TSB, where decrease in TSB level led to increase in CD19+ lymphocytes percentage (P-value<0.05). These results were in line with Khan and Poduval, who demonstrated that unconjugated hyperbilirubinemia has an inhibitory effect on CD19 B cells which could be a result of induction of necrosis and apoptosis in mature immune cells.14 But they were different from those of Yahia et al. and Rami et al., who reported that neonatal hyperbilirubinemia did not influence DNA damage and apoptosis in peripheral blood lymphocytes in full term neonates.17,19 Our results also disagreed with Karabayir et al. who found that there was no significant effect of hyperbilirubinaemia on lymphocyte subgroups.20
In our study, upon comparing patients before phototherapy and untreated control group as regard CD19+, CD4+ and CD8+ lymphocyte percentage, there was a statistical difference only in CD19+ lymphocyte percentage (P<0.05). Kurt et al. and Karabayir et al., stated that no significant difference was determined in lymphocytes subgroups between icteric babies and the control group before phototherapy.4,20
Our study showed that there was no change in CD19+, CD4+, and CD8+ lymphocyte percentages in term neonates before and after 72h of exposure to phototherapy (P>0.05). Our results agreed with El Rashedy et al. who found no statistically significant difference between lymphocytes subsets before and after 72h of exposure to phototherapy.21 Moreover, our results were in agreement with Kurt et al. who found that all lymphocyte subsets were not statistically significantly decreased by the 72h of exposure to phototherapy, except for the percentage of T lymphocyte subset which was significantly lower in new-borns at 72h of exposure to phototherapy.4 Karabayir et al. noticed a significant increase in CD4+ percentage after eight hours of phototherapy but there was no significant change in lymphocyte subsets 48h after phototherapy.20 Teunissen et al. investigated the consequences of direct exposure of T cells to low doses of ultraviolet B in vitro, he found that two or three days after exposure there was no effect on lymphocyte subsets CD4+ or CD8+.22 Tobin et al. stated that during and after narrow band ultraviolet B (UVB) treatment, there were no differences in lymphocyte subsets.23
In our study on comparing the ratio between CD4/CD8 ratio among the studied groups and among patients before and after 72h of exposure to phototherapy there was no statistical difference (P>0.05). These results concurred with Karabayir et al. who also found that there was no change in CD4/CD8 ratio among patients or among the studied groups.20
Before phototherapy, the median value of WBCs in patients was (11.05) and untreated control group levels (8.4) (P-value 0.013) and LC was (4.2) in patients and (5.0) in controls (P-value 0.005). While After 72h of exposure to phototherapy, WBCs decreased to (10.5) and LC increased to (4.6) to become closer to the untreated control group levels of WBCs (8.4) and LC (5.0) (P-value>0.5) for both; so phototherapy restored WBC and LC counts to near the untreated control group levels. Our results disagreed with Jahanshahifard et al. who found that WBC counts rose significantly with phototherapy. The increase in WBC may be due to stress of admission or beginning of infection.24
The follow-up of patients for a period of six months after their discharge showed that there was no increase in frequency of infections or need for hospitalisation. This did not agree with Ustain et al. who studied the effect of neonatal jaundice and phototherapy on the frequency of first year outpatient visits, and found that there was a small increase in first year outpatient visits rates.25 Also, this did not agree with Kemper et al. who found that there was an increase in the number of hospital visits in the first six months of life.26
In conclusion, our results showed that phototherapy had no effect on lymphocyte subsets after 72h of exposure. Additionally, there was no correlation between exposure to phototherapy and occurrence and frequency of infection or admission to hospital. However, phototherapy decreased the level of TBS which led to increase in the percentage of CD19+ lymphocytes in patients after 72h of exposure and it also restored WBC and LC counts to near the untreated control group levels.
More studies are needed to determine the effect of intensive phototherapy on the immune system. Follow up for occurrence and frequency of infection for a period more than six months may be needed.
Ethical disclosuresConfidentiality of dataThe authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
Protection of human subjects and animals in researchThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.27
Conflict of interestAll authors have no conflict of interest
The work was carried out in the Faculty of Medicine, Cairo University, Clinical Pathology Department.