The aim of this study was to investigate the influence of exercise training on oxidative stress and markers of lung inflammation in children with asthma.
MethodsThirty children aged 8-13 years diagnosed with asthma were enrolled in the study as well as 13 healthy children. One group received only pharmacological treatment and the other group was also enrolled in an exercise programme. Venous blood and 24-hour urine samples were obtained from the children enrolled in the study at the beginning and end of the study. Leukotriene E4 and creatinine levels were measured in the urine and matrix metallopeptidase (MMP-9), endothelin-1(ET-1), malnodialdehyde (MDA), IgE and specific IgE levels were measured in blood samples.
ResultsLeukotriene E4, MDA and MMP9 levels decreased significantly with treatment in both groups (p<0.001). However, ET-1 levels decreased significant only in the exercise group (26.5±3.6 vs 21.3±2.4pg/ml respectively, p=0.001). Moreover, ET-1 levels were found to be significantly lower in the exercise group compared to the only pharmacotherapy group (24.2±3.1 vs 21.3±2.4pg/ml, p=0.007).
ConclusionsPositive influences of exercise training in children with asthma may be mediated by decrease in ET-1 levels.
Children and adults with asthma may avoid exercise because of their respiratory symptoms and they may have reduced exercise tolerance due to the increased sensation of dyspnoea.9,10,20 Exercise programmes in asthma have been demonstrated to be associated with improved fitness levels and quality of life as well as a reduction in the need for medications, fewer visits to the emergency department, and decreased absenteeism from school.6,11,15,26,28 Moreover, physical training was found to increase cardiorespiratory fitness and work capacity in patients with asthma.24 Most of these previous studies have enrolled adult patients but a recent study on children demonstrated that eight weeks of basketball training had beneficial effects on exercise capacity and quality of life.4 However, the results of previous studies about the influence of exercise training on spirometry are contradictory.28 Training may increase exercise capacity without leading to a major change in rest lung function tests.18 The basic pathogenetic mechanisms underlying these changes in asthma with exercise training is unclear.
When oxidative stress overwhelms anti-oxidant mechanisms, reactive oxygen species interact with proteins, lipids and DNA leading to pathological consequences.8 Oxidative stress is thought to initiate airway inflammation; therefore it is an important component of asthma pathogenesis.25 It has been demonstrated that endothelin-1 was associated with oxidative stress in lung diseases like emphysema and that it increased MMP-9 activity leading to destruction of parenchyma.7 Moreover, blockage of ET-1 receptors has been reported to down regulate pulmonary inflammation.7
Therefore, the aim of this study was to investigate the influence of exercise training on levels of MDA as a marker of oxidative stress and on ET-1, MMP-9, leukotriene E4 (LTE4) levels as markers of lung inflammation and damage in children with asthma.
Materials and methodsSubjectsThirty children aged 8-13 years diagnosed with asthma in the Paediatric Allergy and Pulmonology Department were enrolled in the study as well as 13 healthy children. Diagnosis of asthma was based on history of recurrent cough and wheezing with prolonged expiration time which demonstrated clinical reversibility with short acting bronchodilator therapy, beta-2 agonist.16 Healthy children were the ones who presented to the Paediatrics outpatient department for healthy follow-up. History of any chronic disease was absent in the healthy control group and they were examined by a paediatrician to rule out any current disease.
Study designOne group of children with asthma received only pharmacological treatment and the other group was enrolled in an exercise programme as well as the pharmacological treatment. Venous blood and 24-hour urine samples were obtained from the children enrolled in the study, at the beginning and the end of the study. Blood samples were centrifuged for five minutes at 4000rpm. Both blood and urine samples were stored in −80°C until biochemical analysis. Leukotriene E4 and creatinine levels were measured in the urine and MMP9, endothelin, MDA, IgE and specific IgE levels were measured in blood samples.
The study was approved by the Institutional Ethical Review Board and informed consent was taken from the parents and children.
Exercise programmeThe exercise programme was carried out by the Physiotherapy and Rehabilitation Department. Children exercised one hour twice a week on a bicycle for eight weeks. They were called in groups of four. The rate of exercise was determined according to the rest heart rate of the children. Submaximal heart rate was calculated as 50% higher than the rest heart rate. Target heart rate during exercise was calculated as 80% of the submaximal heart rate. Pedalling rate required to reach the target heart rate was determined using a pulse oximeter during exercise. During the eight week exercise programme, 15minutes of warm-up exercise was followed by 45minutes of cycling at the target heart rate.
Biochemical measurementsLtE4 was measured in 24-hour urine samples with ELISA (LTE4 EIA kit, Cayman chemical company, Ann Arbor, MI 48108). Creatinine was measured spectrophotometrically in 24-hour urine sample with the Jaffe method using Cobas Integra 800 (Roche). Serum levels of MMP-9 and endothelin were determined by ELISA (Human MMP-9Assay Kit, Bender MedSystems GmbH, A-1030 Vienna, Austria, Europe, and Endothelin EIA kit, Cayman chemical company, Ann Arbor, MI 48108 respectively). IgE and specific IgE levels were measured with the chemiluminescence method using Immulite 2000. Serum MDA levels were determined via quantification of the pink colour obtained with the reaction of MDA with thiobarbituric acid spectrophotometrically at 532nm.
Statistical analysisStatistical analyses were performed by SPSS 10.0 (Chicago IL) computer program. Groups were compared using the Mann Whitney U test and Wilcoxon signed ranks test for continuous variables. Chi square test was used for frequency comparisons.
ResultsSociodemographic characteristics of the study populationMean age of the children in the asthma group (22 male, 8 female) was 9.8±1.8 and mean age of the children in the control group (6 male, 7 female) was 10.3±2.0 years (p=0.33). All children in the asthma group had positive skin prick tests.
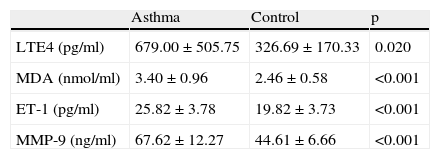
Comparison of LtE4, MDA, ET-1 and MMP-9 levels between asthma and control groupsLeukotriene E4 levels in the asthma group were significantly higher than the control group before initiation of treatment (679.0±505.8 vs 326.7±170.3 respectively, p=0.02). Similarly MDA levels were significantly higher in children with asthma when compared to the controls (3.4±0.9 vs 2.5±0. 6 respectively, p<0.001). ET-1 1 and MMP-9levels were also significantly higher in the asthma group when compared to the control subjects (p<0.001 for both) (Table 1).
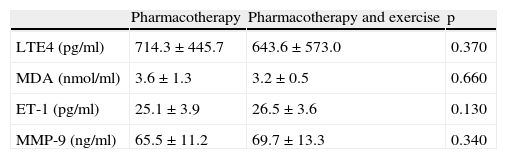
Comparison of levels of LtE4, MDA, ET-1 and MMP-9 in asthma patients who received only pharmacotherapy with the ones who received pharmacotherapy and exercise training before treatmentWhen the group who received only pharmacotherapy was compared with the group who received exercise training as well as pharmacotherapy, no significant differences were detected between levels of LTE4, MDA, ET-1 and MMP-9 (p>0.05 for all) (Table 2).
Comparison of levels of LTE4, MDA, ET-1, and MMP-9 in asthma patients who received only pharmacotherapy with the ones who received pharmacotherapy before treatment.
| Pharmacotherapy | Pharmacotherapy and exercise | p | |
| LTE4 (pg/ml) | 714.3±445.7 | 643.6±573.0 | 0.370 |
| MDA (nmol/ml) | 3.6±1.3 | 3.2±0.5 | 0.660 |
| ET-1 (pg/ml) | 25.1±3.9 | 26.5±3.6 | 0.130 |
| MMP-9 (ng/ml) | 65.5±11.2 | 69.7±13.3 | 0.340 |
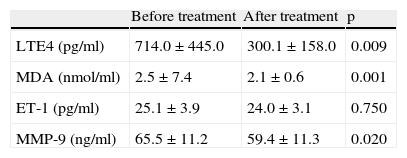
LTE4 levels were found to decrease significantly with treatment in children with asthma who received only pharmacotherapy (714.0±445.0 vs 300.1±158.0 respectively, p=0.009). Similarly MDA and MMP-9 levels were detected to decrease significantly (p=0.001 and p=0.02 respectively). However, ET-1 levels did not change significantly (25.1±3.9 vs 24.0±3.1 respectively, p=0.75) (Table 3).
Levels of LTE4, MDA, ET-1, and MMP-9 in the asthma patients who received only pharmacotherapy before and after treatment.
| Before treatment | After treatment | p | |
| LTE4 (pg/ml) | 714.0±445.0 | 300.1±158.0 | 0.009 |
| MDA (nmol/ml) | 2.5±7.4 | 2.1±0.6 | 0.001 |
| ET-1 (pg/ml) | 25.1±3.9 | 24.0±3.1 | 0.750 |
| MMP-9 (ng/ml) | 65.5±11.2 | 59.4±11.3 | 0.020 |
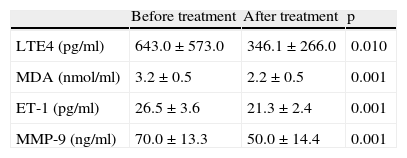
LTE4 levels were found to decrease significantly in children with asthma who received exercise along with pharmacotherapy (643.0±573.0 vs 346.1±266.0 respectively, p=0.01). Similarly, MDA levels decreased from 3.2±0.5 to 2.2±0.5 after therapy (p=0.001). ET-1 and MMP-9 levels were also found to decrease significantly after pharmacotherapy and exercise training (p=0.001 for both) (Table 4).
Levels of LTE4, MDA, ET-1, and MMP-9 in the asthma patients who received exercise training as well as pharmacotherapy before and after treatment.
| Before treatment | After treatment | p | |
| LTE4 (pg/ml) | 643.0±573.0 | 346.1±266.0 | 0.010 |
| MDA (nmol/ml) | 3.2±0.5 | 2.2±0.5 | 0.001 |
| ET-1 (pg/ml) | 26.5±3.6 | 21.3±2.4 | 0.001 |
| MMP-9 (ng/ml) | 70.0±13.3 | 50.0±14.4 | 0.001 |
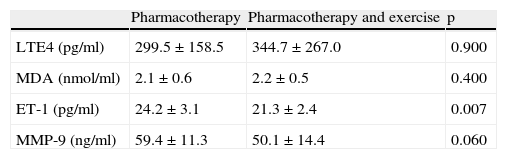
LTE4, MDA, and MMP-9 levels were found to be similar after treatment in children with asthma who received only pharmacotherapy and who received exercise training along with pharmacotherapy (p>0.05 for all). However, ET-1 levels were found to be significantly lower in children who received exercise training as well as pharmacotherapy compared to the ones who received only pharmacotherapy (24.2±3.1 vs 21.3±2.4, p=0.007) (Table 5).
Comparison of levels of LTE4, MDA, ET-1, and MMP-9 in asthma patients who received only pharmacotherapy with the ones who received pharmacotherapy after treatment.
| Pharmacotherapy | Pharmacotherapy and exercise | p | |
| LTE4 (pg/ml) | 299.5±158.5 | 344.7±267.0 | 0.900 |
| MDA (nmol/ml) | 2.1±0.6 | 2.2±0.5 | 0.400 |
| ET-1 (pg/ml) | 24.2±3.1 | 21.3±2.4 | 0.007 |
| MMP-9 (ng/ml) | 59.4±11.3 | 50.1±14.4 | 0.060 |
Asthma, which is one of the most common chronic diseases of childhood, may be associated with fear of exercising due to increased sensation of dyspnoea.5,9 Since functional status of the child influences his or her quality of life to a great extent, it is important to consider this problem during treatment. Asthma severity is an important characteristic to consider when advising patients to exercise. However, most cases of childhood asthma are mild-moderate and it has been demonstrated in adult patients that they achieve maximal heart rate similar to control subjects during exercise.9 Additionally, exercise training has been shown to improve FEV1 levels, mean maximal oxygen uptake as well as the subjective exertion responses.9 Nevertheless, previous studies have not identified the exact pathogenetic mechanism underlying these changes with exercises in children with asthma. Therefore, the aim of this study was to evaluate the influence of exercise training on oxidant stress, inflammation and lung parenchyma destruction parameters in children with asthma.
Leukotriene E-4, which is a cysteinyl leukotriene, may be considered as a biomarker of asthma.13 It is the stable end product of the arachidonic acid metabolism and therefore can be measured.13 It has been reported that a decrease in urinary LTE4 levels was associated with an increase in FEV1 levels.12 Moreover, another research demonstrated that as daily urinary excretion of LTE4 increased, so FEV1 decreased in children with asthma.23 Therefore, LTE4 may be considered as a marker of airflow limitation.12 Moreover, LTE4 has been considered to be one of the main inflammatory mediators of exercise-induced asthma attacks.2 Levels of LTE4 were found to be significantly higher in children with asthma when compared to the healthy children in our study. This finding is in concordance with previous studies that demonstrated correlation of this biomarker with airflow obstruction.12,23 Moreover, LTE4 levels were found to decrease significantly with treatment in children with asthma. But end values in the groups who received only pharmacotherapy and who received exercise training as well as pharmacotherapy was similar. Therefore, exercise was not thought to have a significant influence on urinary LTE4 levels.
Reactive oxygen species generated depleted intracellular glutathione leading to oxidation of membrane phospholipids.27 Malondialdehyde is one of the endogenously generated aldehydic lipid peroxidation products of membrane lipid peroxidation during this process.27 Previous studies have reported that MDA levels decreased somewhat but remained higher than healthy controls even after one to three months of inhaled corticosteroid treatment in patients with asthma, indicating the requirement of longer term treatment to observe values similar to healthy subjects.1,21 Similar to these previous studies, MDA levels in children enrolled in our study were higher than controls at the beginning and improved significantly in both groups of pharmacotheraphy alone and associated with exercise training at the end of eight weeks. However, the levels were similar between these two groups indicating that the addition of exercise training did not influence these levels significantly.
Matrix metalloproteinases are proteolytic enzymes that degrade all components of extracellular matrix and play a role in many physiological and pathological processes.14 MMP-9, which is the most abundantly found MMP in asthmatic subjects, has been shown to play an important role in airway inflammation in asthma.3,14 MMP-9 might also have been proposed to modulate airway remodelling in asthma by activating Transforming growth factor B, increasing collagen synthesis, or by increasing angiogenesis.3 Serum MMP-9 levels in children with asthma were found to be higher than their healthy peers in our study as expected from the explanations above and the levels decreased with treatment in both treatment arms. However, end-treatment values were not different between the group which received exercise training and the one which received exercise training. This might indicate that exercise training does not have an influence on MMP-9 levels.
There are many actions of ETs, one which is smooth muscle contraction and immune system influenced.22 Since lung is the primary organ of ET metabolism, ETs play an important role in asthma.22 ET-1 was reported to increase smooth muscle mass and induce IL-6 in asthma.19 It has been shown in a murine model of asthma that antagonism of ET-A receptor which has a high affinity for ET-1, upregulates lymphocyte infiltration and decreases eosinophils, hyperreactivity and mucus.17 Additionally ET-1 levels in exhaled breath condensate have been reported to be correlated with asthma severity.30 Moreover, ET-1 release from the epithelium was proposed to play an important role in airway inflammatory response after exercise in patients with exercise induced bronchoconstriction.29 The results of our study which demonstrated higher ET-1 levels in children with asthma compared to the healthy one were concordant with the above characteristics of ET-1. Treatment led to a significant decrease in these levels. Moreover, ET-1 levels at the end of the study were lower in the group who received exercise training compared to the ones who received only pharmacotherapy. This is an important finding suggesting that the positive influences of exercise training on asthma might be modulated by its influence on ET-1 at least to some extent.
The major limitation of our study was the small number of subjects enrolled. However, this is a preliminary study which will be followed by more extensive studies about the influence of exercise training on childhood asthma. Moreover, measurement of MDA, MMP-9 and ET-1 in bronchoalveolar lavage would be more informative of levels of these markers of inflammation. However, ethical issues precluded us from taking bronchoalveolar lavage from children with asthma.
In conclusion, the results of our study indicate that children with asthma can undergo exercise training under medical supervision and the positive influences of exercise training may be mediated by the decrease in ET-1 levels.
Conflict of interestNone of the authors have conflict of interest to declare.