Among the preventive strategies for lowering the incidence of upper respiratory tract infections (URTI) and acute diarrhoea episodes, two of the most common diseases in children, zinc supplementation has received special interest. However, there is a need for additional studies that determine the preventive effects of different doses of zinc on URTI and diarrhoeal disease episodes in children.
MethodsIn a randomised, triple-blind clinical trial, we evaluated the efficacy of 12 months of daily zinc supplementation in the incidence of URTI and acute diarrhoea in a population of healthy children aged between 6 and 12 months living in Bogota, Colombia. The outcomes analysed were incidence of URTI, acute diarrhoeal disease episodes, and side effects of the interventions.
ResultsBetween 2010 and 2013, a total of 355 children underwent randomisation, with 174 assigned to the zinc supplementation group and 181 to the control group. In the multivariate analyses, having been randomised to the non-supplemented control group (IRR 1.73, 95% CI 1.52–1.97, p<0.001), and nursery attendance (IRR 1.41, 95% CI 1.07–1.87, p=0.016) were independently linked to the number of URTI. Likewise, having been randomised to the non-supplemented group (IRR 1.43, 95% CI 1.20–1.71, p<0.001), and lower socioeconomic status (IRR 1.86, 95% CI 1.11–3.13, p=0.018) were independently associated to the number of diarrhoeal disease episodes.
ConclusionsDaily supplementation of 5mg of zinc during 12 months significantly decreased the incidence of URTI and diarrhoeal disease episodes in a healthy population of children aged between 6 and 12 months.
Upper respiratory tract infection (URTI) in children is one of the most common reasons for paediatric consultations in primary care and the most common illness resulting in a decreased attendance of school.1 Although the great majority of URTIs are self-limiting conditions and the overall risk of complications is small, they impose a great health and economic burden due to their extremely high frequency. Considering both the frequency and the mean duration of URTI symptoms, it has been estimated that an average child may have symptoms of URTI for nearly six months in the year.2 Likewise, although acute diarrhoea in children is a very common, rarely life-threatening condition, it has been considered the second leading cause of death due to infections among children aged less than five years old worldwide.3 Targeted interventions to reduce the incidence and the impact of respiratory tract infections and episodes of acute diarrhoea have been considered as a necessary strategy for achieving one of the eight Millennium Development Goals, namely the reduction by two-thirds of the under-five mortality rate between 1990 and 2015.4
Among the preventive strategies for lowering the incidence of respiratory tract infections and acute diarrhoea episodes, zinc supplementation has received special interest.5 Zinc is an important trace element that must be included in the diet, because the body can neither produce it nor has adequate mechanism for storing or releasing it in case of decreased intake or increased need.6 Zinc is an essential micronutrient that has effect on multiple organs and systems in the human body, and is involved in over 300 biological functions, including gene expression, apoptosis and synaptic signs.7 Zinc deficiency, even in a low grade, may be associated with both innate and acquired immune system dysfunction,8 and has direct effects on the gastrointestinal tract which can lead to an increased clinical severity of acute gastrointestinal infections.9 This last fact is especially important for children living in low and middle-income countries (LMIC) because of the high prevalence of zinc deficiency reported in these countries10 (in Colombia it is estimated to be 43.3% in children under 5 years11), and the increased zinc demand that children usually have, especially during periods of rapid growth.12 Zinc supplementation is an effective and potentially highly cost-effective strategy that has been studied as an intervention for the treatment and prevention of respiratory tract infections and diarrhoea, especially among children living in LMIC.13,14 A recent Cochrane review that aimed to assess the efficacy of zinc supplementation for preventing mortality and morbidity in children aged six months to 12 years of age, concluded that supplementation reduced diarrhoea morbidity, including the incidence of diarrhoea resulting from all causes, with less precise results for respiratory tract infections.5 The duration of zinc supplementation in the included studies ranged between less than two months and 12 months, and the supplement dose ranged between a daily dose of less than 5mg and 20mg or more. However, this Cochrane review did not include URTI data, and concluded that further research should determine the optimal characteristics of an intervention such as supplement dose. Accordingly, there is a critical need for additional studies that determine the preventive effects of different doses of zinc on URTI and diarrhoeal disease episodes. The latter is essential for planning the implementation of specific measures aimed to decrease the burden of URTI and acute diarrhoea among children living in LMIC such as the initiation of zinc supplementation and food fortification programs.
The aim of the present study was to evaluate the efficacy of 12 months of daily zinc supplementation on the incidence of URTI and diarrhoeal disease episodes in a population of children living in Bogota, a city located in a LMIC.
MethodsThe study site and populationThe study was conducted at Javesalud, a teaching Medical Centre located in the metropolitan area of Bogota, Colombia (2650 metres above sea level). Parents/caregivers of healthy children aged between 6 and 12 months evaluated in our general paediatric service from September 2010 to December 2013 were invited to participate in the study. Parents/caregivers of participating children were Spanish speakers, with widely varied educational backgrounds and socioeconomic status but with at least five years of elementary school. Exclusion criteria included refusal of consent, parental refusal to continue therapy, inadequate medication adherence, chronic lung disease, asthma, persistent diarrhoea, atopic dermatitis, chronic malnutrition (stunting), congenital heart disease, congenital malformations, or prematurity. Children who had been given multivitamins and/or zinc supplements within the previous three months were also excluded.
The study protocol was approved by the Pontificia Universidad Javeriana and Javesalud Research Ethics Committees in accordance with the declaration of Helsinki on the use of human material for research.
Study design and proceduresWe conducted a randomised, triple-blind clinical trial designed to evaluate the efficacy of 12 months of daily zinc supplementation in the incidence of URTI and acute diarrhoea in a population of healthy children aged between 6 and 12 months. Once enrolled in the study, children were assigned randomly to the active supplementation group or to the non-supplemented control group, and observed for 12 months. The randomisation sequence was generated by simple randomisation by one investigator not involved in patient enrolment, using a computer random number generator.
Children in the active supplementation group received 5mg of zinc oxide plus 525mg of calcium carbonate plus 70UI of vitamin D3 (Kidcal®), and children in the non-supplemented control group received 525mg of calcium carbonate plus 70UI of vitamin D3. The mixtures of the active supplementation and the non-supplemented control groups were prepared as syrups and packaged in unbreakable bottles by the Pharmacy Department of Farma de Colombia SA Pharmaceutical (Bogota, Colombia). The mixture of the active supplementation and the non-supplemented control syrups had similar appearance, colour, odour, consistency and fruit flavour. The syrup bottles had identical external appearance and were sequentially numbered according to the randomisation code. None of the participants, parents/caregivers, study team members, or the investigators involved in the assessment of the outcomes, or those involved in data entry or analysis were aware of the assigned treatment of the patients. The randomisation code was known only to the pharmacist and was kept sealed until the data analyses were finished.
In baseline assessment, we used standardised forms to collect demographic data of children (age, gender) and their respective parents/caregivers (highest level of education, socioeconomic status – SES). In Colombia, SES is typically broken into six categories, ranging from 1 to 6 with a higher category indicating better SES, based on geographical location in the city and income. In addition, at baseline and in each of the following assessments, we collected information about the presence of breastfeeding for at least six months, nursery attendance, and food records.
Upon completion of baseline assessment, the children's parents/caregivers were instructed to administer 3.5mm of the assigned syrup at home once daily during 12 months, repeating a dose if the infant vomited within 30min. Likewise, all parents/caregivers were instructed about the importance of not giving their children a diet containing fibre or phytates such as cereals, nuts, and legumes, due to their binding to dietary zinc, preventing its proper absorption.15 During the 12 month check-up period, all children had a complete clinical examination bimonthly in our general paediatric service, and the parents/caregivers were questioned about the presence or absence of respiratory and diarrhoeal disease symptoms in their children. Likewise, parents/caregivers in both groups were contacted monthly by telephone to determine their children's morbidity experience in the previous four weeks, including the number of URTI and diarrhoeal disease episodes.
Medication adherence was measured by medication measurement, documenting the amount of syrup bottles dispensed and returned at follow-up visits.
Outcomes definitionsUpper respiratory tract infections (URTI)URTI was considered as the main measure of the outcome. The presence of an URTI was defined as the presence of cough, itchy or sore throat, runny or stuffy nose, sneezing, watery eyes, slight body aches and/or mild headache, with or without low-grade fever or feeling feverish. If seven or more days had passed since the resolution of the previous URTI, it was considered as a new episode of URTI.
DiarrhoeaDiarrhoea was defined as the presence of three or more liquid, semiliquid, or watery stools in a 24-h period or less. It was considered that there were two different episodes of diarrhoea when the dates of the episodes were separated by three or more days, with a recovery period in between.
Side effectsSide effects were defined as the occurrence of abdominal pain, vomiting, constipation and allergies that first occurred or became worse after treatments were started.
Statistical analysisContinuous variables are presented as mean±standard deviation (SD) or median interquartile range, whichever is appropriate. Categorical variables are presented as numbers (percentage). Differences between continuous variables were analysed using the unpaired t test or Wilcoxon's signed rank test, whichever was appropriate. Associations between categorical variables were analysed using Chi-square test or Fisher's exact test, whichever was appropriate. To identify predictor variables independently linked to the number of URTI and diarrhoeal disease episodes, we fitted Poisson regression models with robust variance.16 Regression results are reported as incident rate ratios (IRR) and their respective 95% confidence intervals (CI).
Sample size was calculated using the software TAMAMU 1.1,17 based on an expected mean difference of 1 URTI per year between the two groups, a standard deviation between the difference of the means of 3.0, a statistical significance level of 0.05, and a power of 80%. The calculation was also based on a loss to follow-up of 20% of patients. Based on these calculations, we planned to enrol at least 339 patients.
All statistical tests were two-tailed, and the significance level used was p<0.05. The data were analysed with the Statistical Package Stata 12.0 (Stata Corporation, College Station, TX, USA).
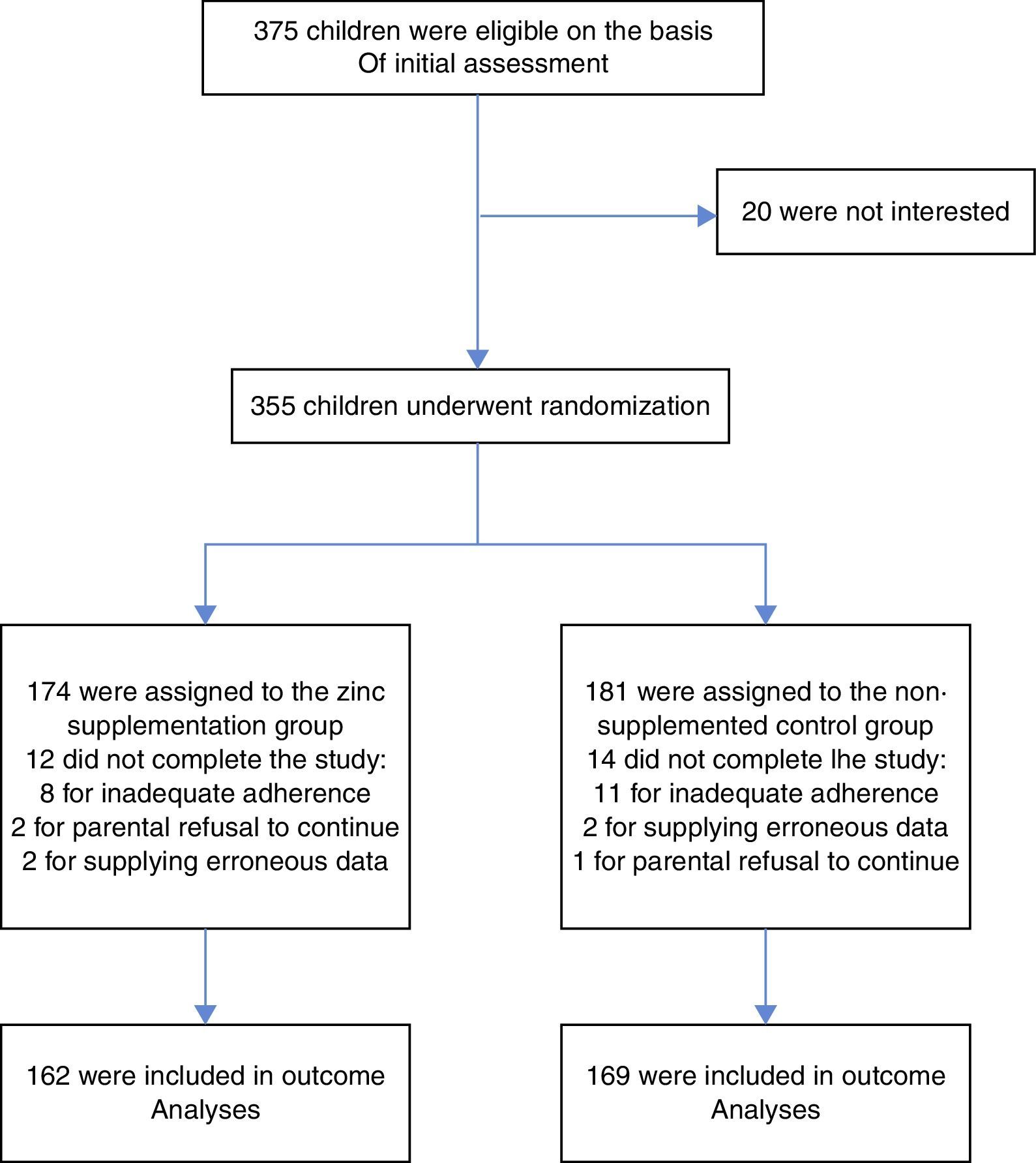
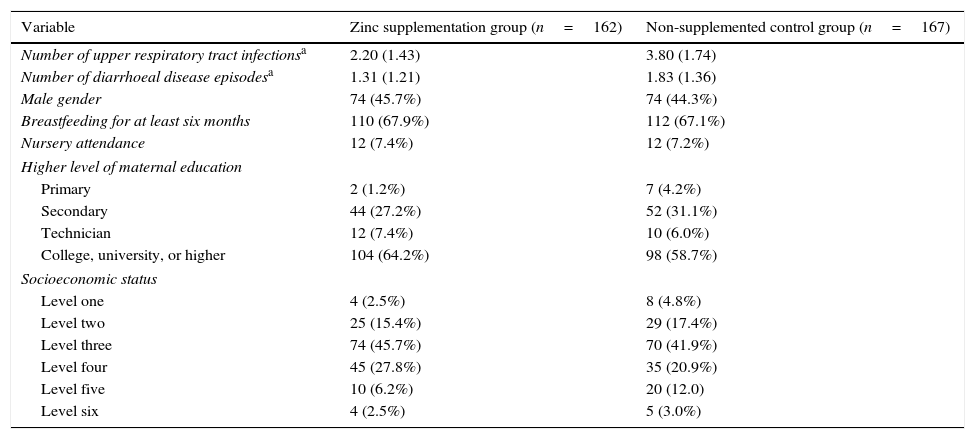
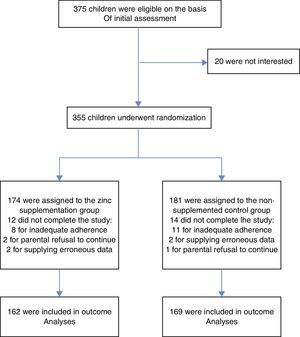
ResultsStudy overview and baseline characteristicsBetween September 21, 2010, and December 21, 2013, a total of 355 children underwent randomisation. Of those randomised, 162 (93.1%) children in the active supplementation group and 167 (92.3%) children in the non-supplemented control group completed all the follow-up visits. Fig. 1 shows the flow diagram of the participants and the reasons for not completing the study, according to treatment group. There was no significant difference in the proportion of patients who did not complete the study between the two treatment groups (p=0.78). There were no significant differences in any of the predictor variables between the two treatment groups. Table 1 shows the patients and parents/caregivers demographical and clinical characteristics at baseline according to study group. Both groups were comparable in terms of age, gender, level of maternal education, socioeconomic status, presence of breastfeeding, nursery attendance, and vaccinations.
Baseline characteristics of patients who completed the study, according the treatment group.
| Variable | Zinc supplementation group (n=162) | Non-supplemented control group (n=167) |
|---|---|---|
| Number of upper respiratory tract infectionsa | 2.20 (1.43) | 3.80 (1.74) |
| Number of diarrhoeal disease episodesa | 1.31 (1.21) | 1.83 (1.36) |
| Male gender | 74 (45.7%) | 74 (44.3%) |
| Breastfeeding for at least six months | 110 (67.9%) | 112 (67.1%) |
| Nursery attendance | 12 (7.4%) | 12 (7.2%) |
| Higher level of maternal education | ||
| Primary | 2 (1.2%) | 7 (4.2%) |
| Secondary | 44 (27.2%) | 52 (31.1%) |
| Technician | 12 (7.4%) | 10 (6.0%) |
| College, university, or higher | 104 (64.2%) | 98 (58.7%) |
| Socioeconomic status | ||
| Level one | 4 (2.5%) | 8 (4.8%) |
| Level two | 25 (15.4%) | 29 (17.4%) |
| Level three | 74 (45.7%) | 70 (41.9%) |
| Level four | 45 (27.8%) | 35 (20.9%) |
| Level five | 10 (6.2%) | 20 (12.0) |
| Level six | 4 (2.5%) | 5 (3.0%) |
The number of URTI were significantly lower among the children randomly assigned to the active supplementation group compared to those assigned to the non-supplemented control group, during the 12 months of follow-ups (2.20±1.43 vs. 3.80±1.74, p<0.001). Likewise, the number of diarrhoeal disease episodes were significantly lower in children assigned to the active supplementation group than in those assigned to the non-supplemented control group (1.31±1.21 vs. 1.83±1.33, p<0.001).
Regarding the presence of side effects, the active supplementation group was associated with a non-significant increase in transient constipation compared to the non-supplemented control group. One child in the non-supplemented control group and none in the active supplementation group presented vomiting episodes. No other side effects were reported during the follow-up period.
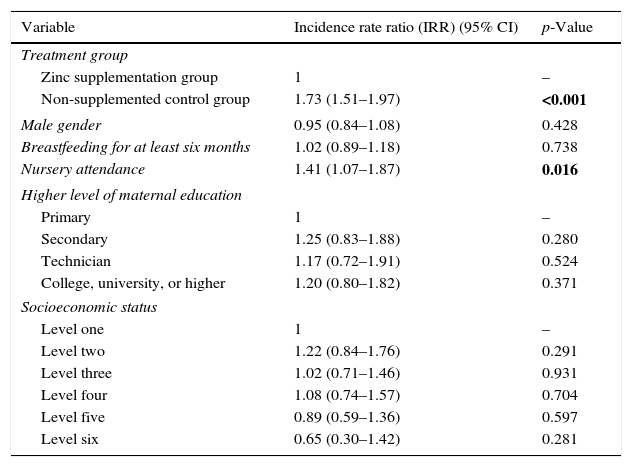
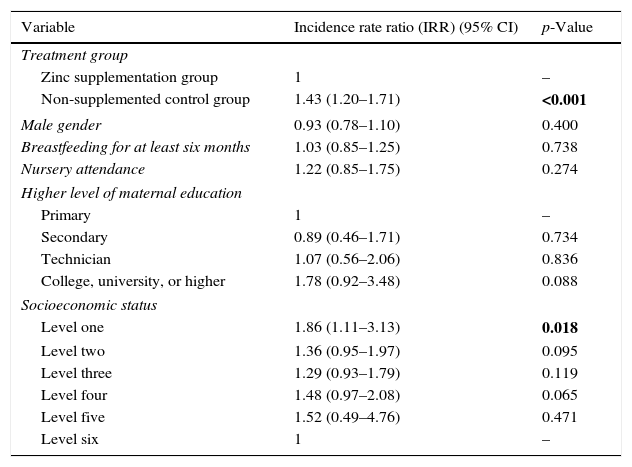
Predictors of the number of URTI and diarrhoeal disease episodes in multivariate analysesIn the multivariate analyses, after controlling for gender, level of maternal education, socioeconomic status, and presence of breastfeeding for at least six months, having been randomised to the non-supplemented control group (IRR 1.73, 95% CI 1.52–1.97, p<0.001), and nursery attendance (IRR 1.41, 95% CI 1.07–1.87, p=0.016) were independently linked to the number of URTI (Table 2). Likewise, when analysing the number of diarrhoeal disease episodes as an outcome, we found that after controlling for gender, level of maternal education, presence of breastfeeding for at least six months, and nursery attendance, having been randomised to the non-supplemented group (IRR 1.43, 95% CI 1.20–1.71, p<0.001), and lower SES (IRR 1.86, 95% CI 1.11–3.13), p=0.018 were independently associated to the number of diarrhoeal disease episodes (Table 3).
Predictors of the number of upper respiratory tract infections in multivariate analysis.
| Variable | Incidence rate ratio (IRR) (95% CI) | p-Value |
|---|---|---|
| Treatment group | ||
| Zinc supplementation group | 1 | – |
| Non-supplemented control group | 1.73 (1.51–1.97) | <0.001 |
| Male gender | 0.95 (0.84–1.08) | 0.428 |
| Breastfeeding for at least six months | 1.02 (0.89–1.18) | 0.738 |
| Nursery attendance | 1.41 (1.07–1.87) | 0.016 |
| Higher level of maternal education | ||
| Primary | 1 | – |
| Secondary | 1.25 (0.83–1.88) | 0.280 |
| Technician | 1.17 (0.72–1.91) | 0.524 |
| College, university, or higher | 1.20 (0.80–1.82) | 0.371 |
| Socioeconomic status | ||
| Level one | 1 | – |
| Level two | 1.22 (0.84–1.76) | 0.291 |
| Level three | 1.02 (0.71–1.46) | 0.931 |
| Level four | 1.08 (0.74–1.57) | 0.704 |
| Level five | 0.89 (0.59–1.36) | 0.597 |
| Level six | 0.65 (0.30–1.42) | 0.281 |
The significance of “bold values” indicates p value≤0.05.
Predictors of the number of diarrheal disease episodes in multivariate analysis.
| Variable | Incidence rate ratio (IRR) (95% CI) | p-Value |
|---|---|---|
| Treatment group | ||
| Zinc supplementation group | 1 | – |
| Non-supplemented control group | 1.43 (1.20–1.71) | <0.001 |
| Male gender | 0.93 (0.78–1.10) | 0.400 |
| Breastfeeding for at least six months | 1.03 (0.85–1.25) | 0.738 |
| Nursery attendance | 1.22 (0.85–1.75) | 0.274 |
| Higher level of maternal education | ||
| Primary | 1 | – |
| Secondary | 0.89 (0.46–1.71) | 0.734 |
| Technician | 1.07 (0.56–2.06) | 0.836 |
| College, university, or higher | 1.78 (0.92–3.48) | 0.088 |
| Socioeconomic status | ||
| Level one | 1.86 (1.11–3.13) | 0.018 |
| Level two | 1.36 (0.95–1.97) | 0.095 |
| Level three | 1.29 (0.93–1.79) | 0.119 |
| Level four | 1.48 (0.97–2.08) | 0.065 |
| Level five | 1.52 (0.49–4.76) | 0.471 |
| Level six | 1 | – |
The significance of “bold values” indicates p value≤0.05.
The present study showed that daily supplementation of 5mg of zinc oxide during 12 months, decreased the incidence of URTI and diarrhoeal disease episodes in a population of healthy children aged between 6 and 12 months living in Bogota, a city located in a LMIC. Additionally, besides zinc supplementation, nursery attendance and lower SES were also associated with the number of URTI and diarrhoeal disease episodes, respectively.
The current findings add to a growing body of literature on the preventive effects of zinc supplementation on respiratory tract infections and diarrhoeal disease episodes, by providing additional information on intervention characteristics such as supplement dose and the duration of its administration. We are confident that our results might be a first step towards the inclusion of preventive zinc supplementation in public health programs to reduce respiratory and diarrhoeal morbidity in Colombia and probably other similar LMIC. This is because the high prevalence of zinc deficiency reported in LMIC, the high burden of disease attributable to respiratory infections and diarrhoeal disease in these countries; including the fact that zinc supplementation is an easily implemented and inexpensive intervention. Although URTI and diarrhoeal disease episodes are usually benign and self-limited conditions, their extremely high prevalence in children and the possibility of evolution towards a more serious disease means that prevention interventions aimed at reducing their incidence can be considered as a public health priority.
Our results have a number of similarities with those reported by other authors, who have evaluated the preventive effects of zinc supplementation on respiratory tract infections and diarrhoeal disease episodes. With respect to its effect on respiratory tract infections, Kurugol et al. determined the efficacy of prophylactic administration of 15mg of zinc during seven months in reducing the occurrence of the common cold in 200 healthy children aged between two and 10 years. The authors reported that the mean number of colds in the zinc group was significantly less than in the placebo group. They also found significant differences in the mean cold-related school absence, shorter mean duration of cold symptoms, and decreased total severity scores for cold symptoms, favouring the zinc-treated group.18 Likewise, Vakili et al., in a randomised double-blind, placebo-controlled trial, determined whether supplementation of 10mg of zinc could reduce frequency rate and duration of cold during cold season 200 school aged children living in a low socioeconomic region. They found a significant decrease in common cold incidence among the zinc-supplemented group compared to the placebo group. Other outcomes were also significantly different between the two groups favouring the zinc-treated group, such as the average number of days of absence from school and the need for administration of antibiotics for bacterial infections.19 These two latter studies were summarised in a recent Cochrane review, reporting a significant IRR of developing a cold in children who received zinc intervention compared with those who received placebo (0.64, 95% CI 0.47–0.88, p=0.006).20 These findings partially correspond with those reported in a double-blind randomised placebo-controlled trial published after this Cochrane review. In this study authors assessed the efficacy of 15mg (zinc bis-glycinate) given once a day for three months during the winter season to healthy school children aged 8–13 years to prevent symptoms of the common cold. Although zinc supplementation was not associated with a reduction in the incidence of the common cold, it was associated with a significant reduction in duration of cough, rhinorrhoea, and the frequency of having two or more symptoms of the common cold.21 Although these studies and our study used variable doses, formulations, and duration of zinc supplementation, and had different follow-up periods, they all have in common that zinc supplementation was associated with a clinically significant impact on the incidence and/or duration of URTI. The great majority of serotypes of Human rhinoviruses, considered as the most frequent agents causing URTI, utilise intercellular adhesion molecule-1 (ICAM-1) for attachment and entry into nasal epithelium cells to exert their effects.22 Among the various mechanisms involved in the antiviral effects of zinc, the ICAM-1 receptor blocking has been considered as one of the most important actions for impacting on incidence and or duration of URTI.23 Our results related to the association between number of URTI and nursery attendance are in line with previous studies that have reported a positive and significant association between nursery attendance and upper respiratory tract morbidity.24,25
Regarding the effect of zinc on prevention of diarrhoeal disease episodes, our results are similar to those reported by Mayo-Wilson et al. In a Cochrane review, the authors assessed the effects of preventive zinc supplementation on diarrhoea morbidity in children aged six months to 12 years compared with no intervention, a placebo, or a waiting list control. The authors found a significant 13% reduction in the incidence of all-cause diarrhoea, a 12% reduction in the prevalence all-cause diarrhoea, a significant combined effect on incidence of severe diarrhoea, a 27% decrease in the incidence of persistent diarrhoea, and a significant 30% reduction in the prevalence of persistent diarrhoea.5 Mechanisms by which zinc exerts its preventive effects on diarrhoea are not fully understood. It has been proposed that zinc plays an important role in modulating the host resistance to infectious agents, thus reducing the risk of diarrhoeal disease episodes. Zinc has been shown to increase the levels of brush border enzymes, and enhance the immune response against intestinal germs, allowing for a better clearance of the pathogens.26 It has been described that by causing a decrease in intracellular cyclic adenosine monophosphate concentration, zinc substantially inhibits toxin-induced cholera.27 It has also been proposed that zinc also exerts its anti-diarrhoeal effects playing a critical role in cellular growth and in the function of the immune system, by its action on metallo-enzymes, polyribosomes, and the cell membrane and cellular function.26 As we found in our study, there are previous studies reporting positive and significant associations between lower SES and increased diarrhoeal morbidity.28,29
We are aware that our research may have three limitations. The first is the failure to assess baseline plasma zinc concentrations. However, zinc deficiency is highly prevalent in children living in LMIC, zinc has been described to be effective even in the absence of zinc deficiency,30 and we used a relatively low dose of zinc in our study. The second is the use of parental/caregivers report of diagnosis of URTI and diarrhoea as opposed to a medical diagnosis of these conditions. However, we used an objective and reproducible case definition for diagnosis of URTI and diarrhoea. Finally, this study was limited by a relatively small and non-representative of sample size. However, despite these shortcomings, we consider that our results go some way towards enhancing our knowledge of the preventive effects of zinc supplementation on respiratory tract infections and diarrhoeal disease episodes in healthy children.
In conclusion, the present study shows that daily supplementation of zinc during 12 months, decreased the incidence of URTI and diarrhoeal disease episodes in a healthy population of infants living in Bogota, a city located in a LMIC. Additionally, besides zinc supplementation, attendance of child care and lower socioeconomic status were also associated with the number of URTI and diarrhoeal disease episodes, respectively.
Ethical disclosuresConfidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Protection of human subjects and animals in researchThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
FundingFarma de Colombia SA Pharmaceutical provided the mixtures of the zinc supplementation and the non-supplemented control syrup bottles. However, the authors had complete independency over the conduct, integrity, and publication of the study.
Conflict of interestThe authors have no conflict of interest to declare.
We thank Nancy Maria Martinez-Rybinska for her editorial assistance. Likewise we thank to Drs Juan Carlos Villar and Juan Guillermo Perez for their help with the preliminary statistical analysis.