The efficacy of corticosteroid has not been thoroughly studied in the treatment of non-allergic rhinitis. This study was designed to compare the efficacy of nasal corticosteroid in patients with allergic rhinitis (AR), and non-allergic rhinitis (NAR).
MethodsThe efficacy of triamcinolone acetonide nasal spray (TANS) on total nasal symptom scores (TNSS), and nasal peak inspiratory flow rate (nPIFR) was studied in a six-week parallel-group trial of NAR (n: 25), and AR (n: 16) patients. Health-related quality of life (HRQoL) and Epworth Sleepiness Scale (ESS) were also analysed.
ResultsThe TNSSs, and symptom scores of conjunctivitis, snoring, and postnasal drainage were significantly improved in both groups, after two and six weeks of treatment. In contrast to AR, patients with NAR had statistically significant improvement in nasal obstruction, and postnasal drainage beginning from two weeks of the treatment. nPIFR slightly increased in both groups. Scores of generic (SF-36), rhinitis specific (MiniRQLQ) and ESS questionnaires generally improved better in AR than NAR. TANS was well-tolerated in AR and NAR groups with minor adverse events including headache, nasal burning, and bitter mouth taste.
ConclusionsOur study disproved the idea of ineffectiveness of corticosteroid treatment in NAR, and showed that triamcinolone acetate may be an alternative drug in the treatment of NAR.
Allergic rhinitis (AR) describes nasal symptoms induced by an immunoglobulin E (IgE) mediated inflammation after allergen exposure, whereas non-allergic rhinitis (NAR) is unrelated to allergy, infection, structural lesions, systemic disease, or drug abuse.1,2 Not only in the presentation of symptoms are AR and NAR often indistinguishable from one another, but also the differential diagnosis of NAR is quite extensive.3 There is no single valid test for the diagnosis of NAR since it is an idiopathic form of rhinitis, and a diagnosis can be made only when all other forms of rhinitis have been excluded.4
Despite its unknown aetiology, there are some suggestions as to whether it is a local allergic (entopy) or a neurogenic response.5,6 Local allergy is related with an inflammatory process which can be resolved by the corticosteroids.7 On the other hand, the nervous system has been reported to play an important role in the mechanism of NAR, suggesting that it is a non-inflammatory type of rhinitis, and corticosteroids will have no effect on the rhinitis symptoms.8,9 Therefore, data about the response to corticosteroids in patients with NAR may help us to highlight its unknown pathology.
Another obscure point in NAR is its treatment with no clear recommendation in guidelines. The treatment studies suggest medications for NAR depending on symptoms of rhinitis.10 Topical sympathomimetic for nasal congestion, topical anticolinergic for rhinorrhea, antihistamine for sneezing are the advised ones.1 Topical fluticasone propionate and azelastine were approved drugs for NAR by Food and Drug Authority, and capsaicin was recommended in NAR in the event of no response to both steroid and antihistamines.11 But in practice, topical corticosteroids are the most prescribed drugs in NAR, although their efficacy in NAR is doubtful except eosinophilic rhinitis (NARES).1 We may explain this with the fact that corticosteroids are known as the most potent anti-rhinitic drugs since they interfere with the inflammatory response by blocking the production of arachidonic acid metabolites, and inhibit the accumulation of inflammatory cells, and vascular permeability.12
Failure in the diagnosis and in the treatment of NAR has significant consequences, including health related quality of life (HRQoL).3 Both AR, and NAR affect HRQoL, predominantly associated with impaired sleep and increased irritability.3,13–15 Therefore, NAR should be treated not only for nasal symptoms but also for a better HRQoL.
The aim of this study was to compare the efficacy of intranasal corticosteroid – triamcinolone acetonide nasal spray (TANS) – in the treatment of rhinitis patients with or without allergy. We also analysed the efficacy of medication on nasal inspiratory flow rate (nPIFR), HRQoL, and sleep disturbance that may interact with rhinitis as well.
MethodsThis single-blind, parallel-group, clinical study was conducted in a university hospital between May 2008 and 2009. The clinical research protocol was approved by the local ethic committee (2008/061), and written informed consent was obtained from the participants. The funding was obtained from Kirikkale University Scientific Research Unit (No: 2007-4).
Selection of patientsThe subjects were evaluated by using medical history, physical examination, skin prick tests (SPTs), blood samples, and paranasal computed tomography (PNCT). Diagnosis and severity of rhinitis was assessed according to guidelines.1 AR was defined as a symptomatic disorder of the nose induced by allergen exposure, and the diagnosis was based on the positivity of SPTs with clinical relevance. NAR was defined as nasal symptoms with unknown pathophysiology. In the diagnosis of NAR, rhinitis types with known aetiology were excluded by using non-allergic triggers (pollution, chemical, olfactory, temperature, weather, work related, and food) or medications in history besides the laboratory. NAR was defined as nasal symptoms with negative SPTs, and fulfilling the exclusion criteria by using non-allergic triggers (pollution, chemical, olfactory, temperature, weather, work related, and food) or medications in history, besides the laboratory.2 Allergy (positive SPT and intradermal test), mechanical obstruction, and sinusitis in PNCT, infection (purulent drainage, and/or high C-reactive protein (CRP) in blood), non-allergic rhinitis with eosinophilic syndrome (>20% eosinophil in nasal smear and/or blood), senile (persistent watery rhinorrhea started in the elderly), atrophic (nasal surgery), hormonal (rhinitis induced by pregnancy or premenstrually), occupational (rhinitis induced by work related irritants), vasomotor (rhinorrhea induced by changes in temperature), and drug induced rhinitis (repeated administration of nasal decongestants) were excluded in NAR group.4 Subjects with a diagnose of systemic diseases, allergen immunotherapy, infection within four weeks, pregnancy or lactating, irritant induced rhinitis, history of anaphylaxis, smokers, and users of antihistamine or anti-inflammatory medication four weeks before the study were excluded.
Skin prick testsSPTs were performed with a battery of common inhalant allergens (ALK, Madrid, Spain). However, in some selected patients (NAR group) intradermal tests were essential to exclude false-negative reactions and to detect relevant allergen(s).
nPIFRNasal congestion was evaluated by using a nPIFR (Clement Clarke International Limited, England) with a face mask, and best of three measurements was used for data analysis in a scale between 20 and 350L/min.
Blood analysisVenous blood samples were analysed for haemogram (flow cytometry, Beckman Coulter), total IgE (electrochemiluminescence, Roche), and C-reactive protein (CRP) (immunoturbudimetric, Roche).
Treatment and nasal symptom scoringPatients with rhinitis were assigned to receive a single dose of TANS (one spray per nostril once daily, 220μ/day) for six weeks. A washout period of at least two weeks was intercalated between patient recruitment and treatment start. The primary efficacy variable was the total nasal symptom score (TNSS), as well as individual symptom scores. Total symptom scoring (TSS) was used as a validated evaluation for the severity of nasal obstruction, itching, rhinorrhea, sneezing, which was graded as ‘0: none, 1: mild, 2: medium, 3: severe’.16 Additional symptoms, adverse events, and nPIFR were also recorded.
HRQoLThe HRQoL questionnaires were rated by the patients at baseline, and after two and six weeks of the treatment. Medical outcomes study 36-item short-form health survey (SF-36), and mini rhinitis quality of life (MiniRQLQ) were used for determining generic and specific HRQoL.17,18 The SF-36 questionnaire includes 36 items, and assesses eight health concepts as measures of physical (PCS) and mental component summary (MCS). SF-36 was rated using 0–100 scoring where higher scores provide a better health. MiniRQLQ was composed of five domains with a total of fourteen questions, and was scored between 0 and 6 which higher score provides deterioration in rhinitis. The Epworth Sleepiness Scale (ESS) was an eight-item questionnaire with scores ranging from 0 to 24. Higher scores indicate greater daytime sleepiness and a cut-off score of >10 represent an abnormal level of daytime sleepiness.19 All the questionnaires were validated in Turkish language.
StatisticsThe statistical analysis was performed using SPSS-15 software. Intergroup comparisons regarding categorical variables were done with χ2-test, and quantitative variables were determined by Student's t-test in treatment groups. The pre-post treatment comparison was done by Wilcoxon test. A value of p<0.05 was regarded as statistically significant.
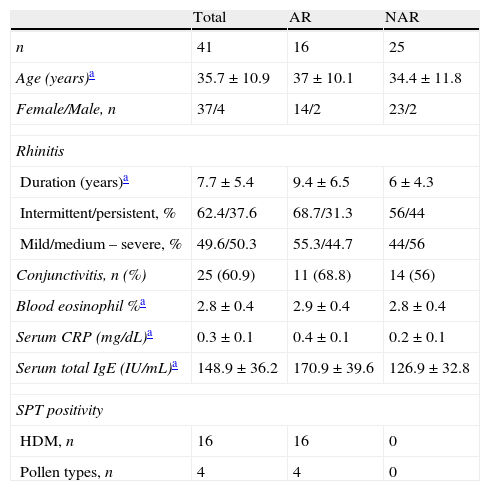
ResultsForty-one patients (37 female/4 male) with a mean age of 35.7±10.9 years (range: 18–56 years) were enrolled, and grouped as NAR (n: 25), and AR (n: 16) (Table 1). Duration of rhinitis was 7.7 years. Both groups had similar ratios of rhinitis characteristics, and frequency of conjunctivitis. Hypereosinophilia and infection were ruled out by using blood eosinophil count, and CRP, which were within normal levels in both groups. Mean serum total IgE level was high only in AR group. All AR patients had a positive response to HDMs, and additionally four of them were sensitive to pollens.
Demographic and rhinitis characteristics of the study group with results of blood tests.
| Total | AR | NAR | |
| n | 41 | 16 | 25 |
| Age (years)a | 35.7±10.9 | 37±10.1 | 34.4±11.8 |
| Female/Male, n | 37/4 | 14/2 | 23/2 |
| Rhinitis | |||
| Duration (years)a | 7.7±5.4 | 9.4±6.5 | 6±4.3 |
| Intermittent/persistent, % | 62.4/37.6 | 68.7/31.3 | 56/44 |
| Mild/medium – severe, % | 49.6/50.3 | 55.3/44.7 | 44/56 |
| Conjunctivitis, n (%) | 25 (60.9) | 11 (68.8) | 14 (56) |
| Blood eosinophil %a | 2.8±0.4 | 2.9±0.4 | 2.8±0.4 |
| Serum CRP (mg/dL)a | 0.3±0.1 | 0.4±0.1 | 0.2±0.1 |
| Serum total IgE (IU/mL)a | 148.9±36.2 | 170.9±39.6 | 126.9±32.8 |
| SPT positivity | |||
| HDM, n | 16 | 16 | 0 |
| Pollen types, n | 4 | 4 | 0 |
AR: Allergic rhinitis, CRP: C-reactive protein, NAR: Non-allergic rhinitis, SPT: Skin prick test, HDM: House dust mites.
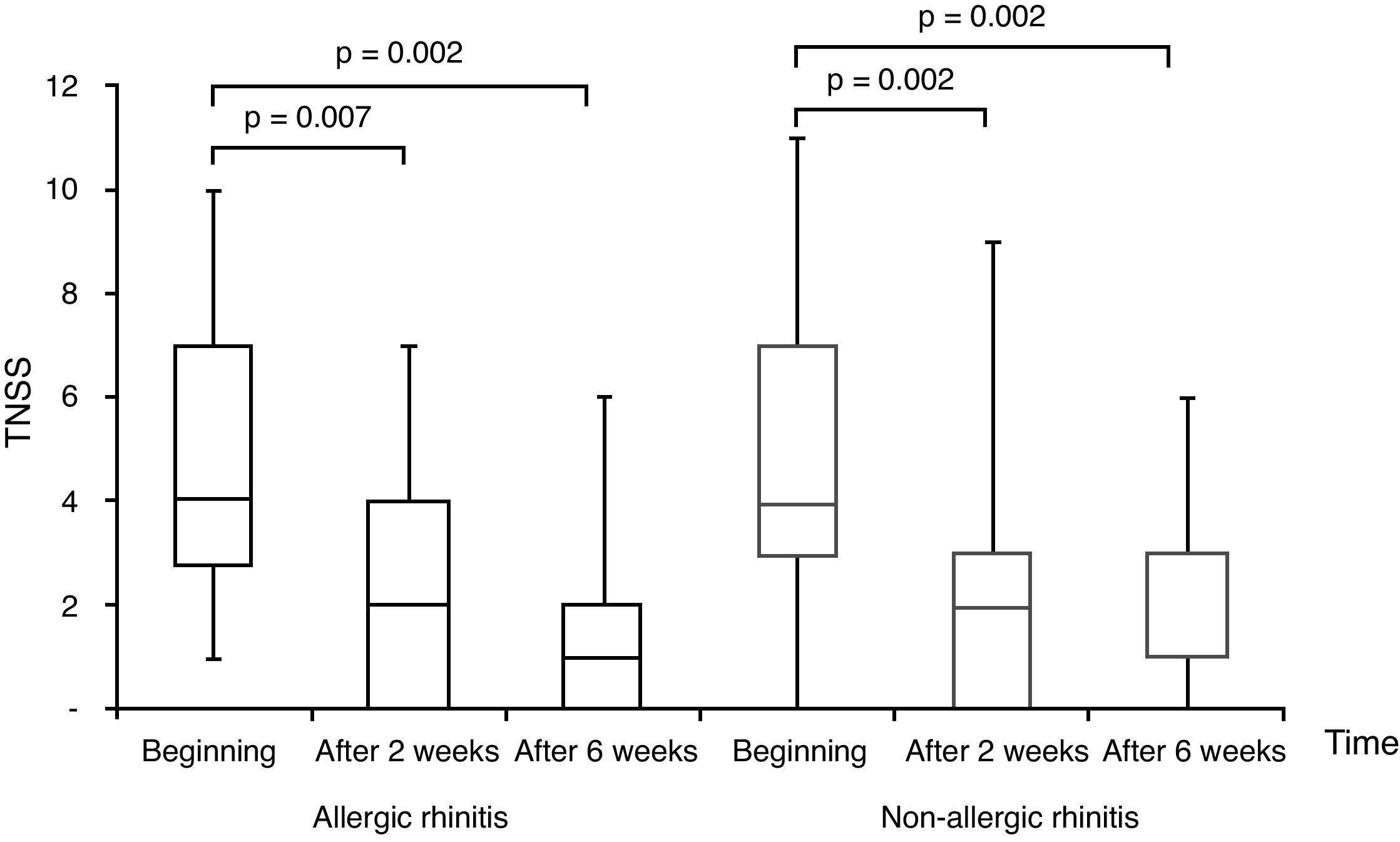
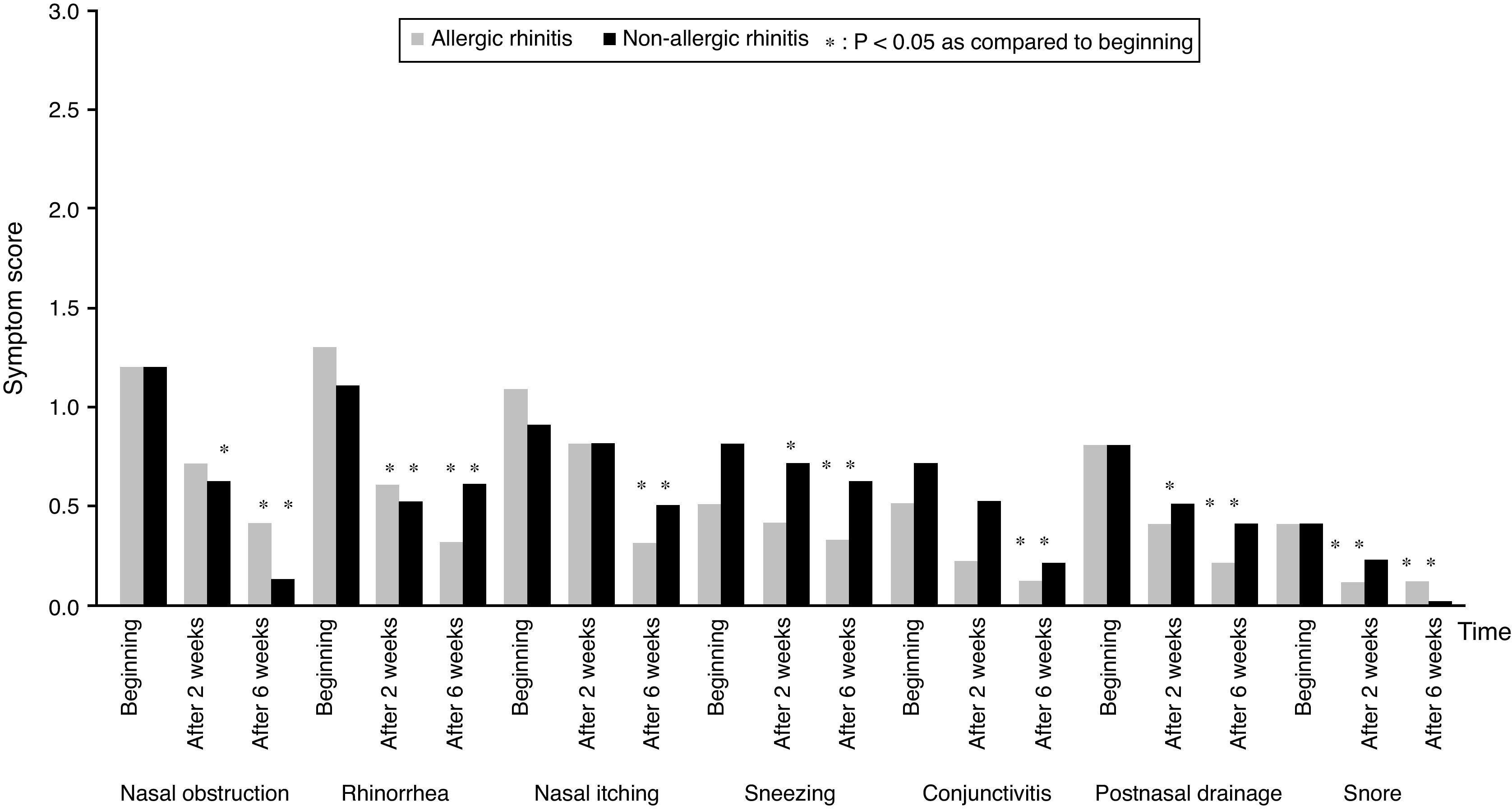
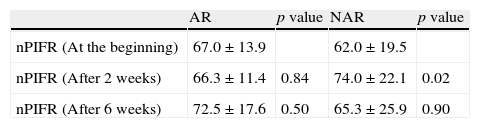
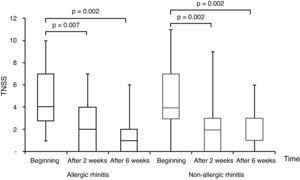
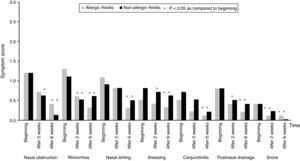
All subjects completed the study protocol. The TNSSs were significantly decreased compared to initial values in both AR, and NAR groups, after two and six weeks of TANS treatment (Fig. 1). In contrast to AR, patients with NAR had statistically significant improvement in nasal obstruction and postnasal drainage beginning from two weeks of the treatment (Fig. 2). The most recovered symptoms at the end of treatment were rhinorrhea, nasal itching, and postnasal drainage in AR, whereas rhinorrhea and nasal obstruction were the most improved symptoms in NAR. TANS was also effective on conjunctivitis, snoring and postnasal drainage in patients with NAR and AR. Even though nPIFR was slightly increased in both groups, a statistically significant increase was observed only in NAR, after two weeks of TANS treatment (p=0.02) (Table 2).
Comparison of nasal peak inspiratory flow rates (nPIFR - L/min) at the beginning and after the treatment with triamcinolone acetonide nasal spray.
| AR | p value | NAR | p value | |
| nPIFR (At the beginning) | 67.0±13.9 | 62.0±19.5 | ||
| nPIFR (After 2 weeks) | 66.3±11.4 | 0.84 | 74.0±22.1 | 0.02 |
| nPIFR (After 6 weeks) | 72.5±17.6 | 0.50 | 65.3±25.9 | 0.90 |
AR: Allergic rhinitis, NAR: Non-allergic rhinitis.
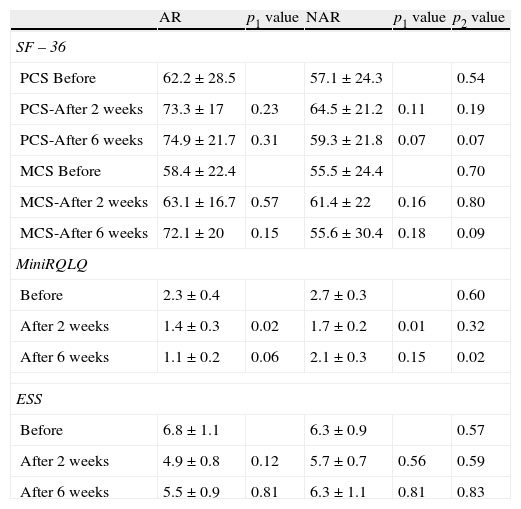
At the beginning, SF-36 and MiniRQLQ scores were similar between the groups, and after the treatment, both scores generally improved better in the AR than the NAR group (Table 3). A statistically significant improvement was observed only in MiniRQLQ scores, after two weeks of TANS, in AR and NAR (p=0.02 and p=0.01), but scores of AR group were better than NAR group, after six weeks of TANS (p=0.02). After the treatment, ESSs slightly decreased in AR, and almost no change happened in NAR (p>0.05, and p>0.05).
Comparison of generic (SF-36) and rhinitis specific (MiniRQLQ) quality of life, and Epworth Sleepiness Scale (ESS) questionnaires between allergic (AR) and non-allergic rhinitis (NAR) with triamcinolone acetonide nasal spray.
| AR | p1 value | NAR | p1 value | p2 value | |
| SF – 36 | |||||
| PCS Before | 62.2±28.5 | 57.1±24.3 | 0.54 | ||
| PCS-After 2 weeks | 73.3±17 | 0.23 | 64.5±21.2 | 0.11 | 0.19 |
| PCS-After 6 weeks | 74.9±21.7 | 0.31 | 59.3±21.8 | 0.07 | 0.07 |
| MCS Before | 58.4±22.4 | 55.5±24.4 | 0.70 | ||
| MCS-After 2 weeks | 63.1±16.7 | 0.57 | 61.4±22 | 0.16 | 0.80 |
| MCS-After 6 weeks | 72.1±20 | 0.15 | 55.6±30.4 | 0.18 | 0.09 |
| MiniRQLQ | |||||
| Before | 2.3±0.4 | 2.7±0.3 | 0.60 | ||
| After 2 weeks | 1.4±0.3 | 0.02 | 1.7±0.2 | 0.01 | 0.32 |
| After 6 weeks | 1.1±0.2 | 0.06 | 2.1±0.3 | 0.15 | 0.02 |
| ESS | |||||
| Before | 6.8±1.1 | 6.3±0.9 | 0.57 | ||
| After 2 weeks | 4.9±0.8 | 0.12 | 5.7±0.7 | 0.56 | 0.59 |
| After 6 weeks | 5.5±0.9 | 0.81 | 6.3±1.1 | 0.81 | 0.83 |
Values: mean±standard deviation, PCS: Physical component summary, MCS: Mental component score, p1: p value between before and after treatment, p2: p value between AR and NAR.
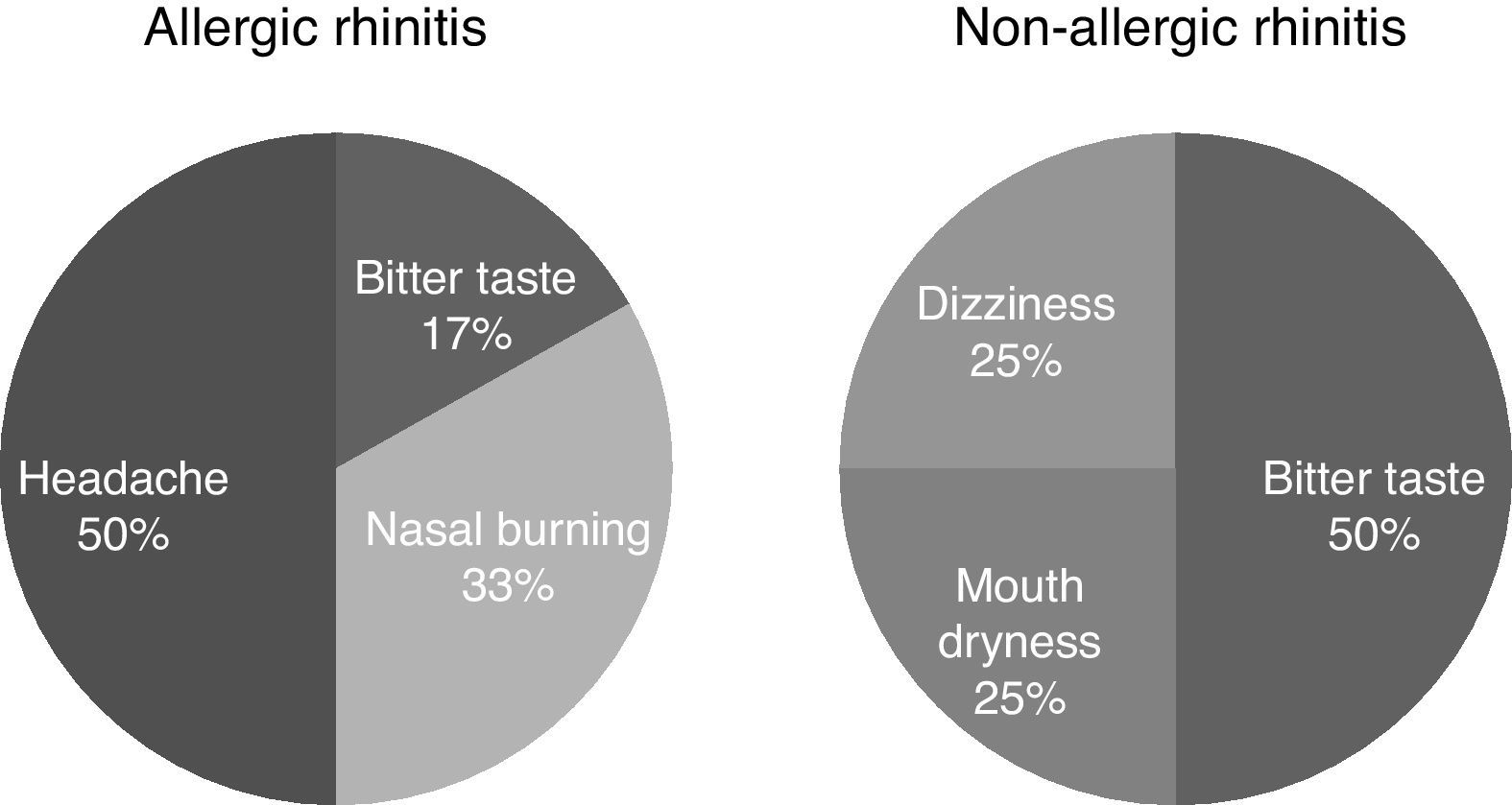
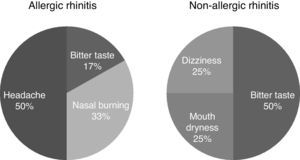
The frequency of adverse events due to TANS was similar between the AR and NAR groups (37.5% and 16%, p=0.15). Although no serious or unexpected events were reported, headache (n: 3), nasal burning (n: 2), and bitter mouth taste (n: 3) were the most frequent ones (Fig. 3).
DiscussionIn this study, we showed that TANS may be indicated for NAR as a choice of topical corticosteroid. Although there was more evidence for the efficacy of TANS on AR in the literature, this study showed that TANS in NAR patients was as effective as AR in terms of TNSS, individual symptom scores, nPIFR, and HRQoL scores.
NAR patients were compared with the AR group including similar demographic and rhinitis characteristics. Patients were treated with nasal corticosteroid for six weeks which was reported to be the optimal time length to show the efficiency of the drug.20 In previous studies, it was shown that patients with NAR had improvement in nasal symptoms after eleven weeks with mometasone furoate,21 after two weeks with budesonide,22 after twenty-eighth days with fluticasone,20 and after two weeks with triamcinolone nasal spray.23 In a previous study, patients with NAR seemed to have a greater benefit in symptom scores than AR patients with two weeks of TANS treatment.23 Similarly, in this study patients with NAR seemed to have a greater improvement than AR patients after two weeks of TANS treatment. However, as a novelty TANS was similarly effective on symptoms in both groups at the end of six weeks. We may speculate that the pathophysiology of NAR may be simpler than AR including less inflammation which is easy to resolve.
TANS was found to be effective on conjunctivitis, snoring and postnasal drainage, besides the nasal symptoms in both rhinitis types. Symptoms with a better improvement observed in this study were also the common ones seen in AR, and NAR. Previously, rhinorrhea, itching and sneezing were reported to be more common in atopic patients,3,24,25 and histamine was shown to trigger these symptoms.26 Furthermore, nasal obstruction and rhinorrhea were reported as primary symptoms of NAR,25 and neurogenic activation was reported to cause rhinorrhea.27
The effect of TANS on nasal symptoms in this study confirms that corticosteroids are effective on symptoms irrespective of aetiology.
In this study, TANS improved nasal airflow as shown by nPIFR in both groups of patients. However the statistical significance of the improvement in nPIFR was not completely parallel with the nasal obstruction. Although the best of three measurements were taken into account, dyscooperation of the patients might have happened as mentioned before.28
Corticosteroids act as an anti-inflammatory drug, independent with the aetiology of rhinitis,11 and the pathophysiology of NAR is poorly understood, but a key component involves nasal allergic or neurogenic activation.5,6 Previous studies about local IgE results in the nasal mucosa of NAR and response to corticosteroid treatment in NAR patients support the existence of allergic inflammation.5,7 On the other hand, there was no improvement in rhinitis symptoms after the treatment of nasal fluticasone in NAR patients,29 and some authors suggest that corticosteroid treatment should not be the first choice for NAR since it had a non-inflammatory neurogenic mechanism.11,30 Recently, it had been shown that neuropeptides had pro-inflammatory effects on the airways which might result in airway hyperactivity and rhinitis.8,31 Furthermore, corticosteroids have been reported to inhibit neurogenic plasma extravasation.12 Therefore in this study, the efficacy of triamcinolone in NAR patients may indicate the inflammatory nature of non-allergic rhinopathy which may be the result of not only allergic activation, but also the effect of neuropeptides.32 Confirming this result, in a previous study, the similarity in the efficacy of intranasal antihistamine and triamcinolone treatment in patients with NAR and AR confirms the existence of a common pathology in both rhinitis types.
Generic and specific HRQoL was improved in both rhinitis patients, but there was a higher improvement in the HRQoL of AR than NAR group after treatment with TANS. Previously, HRQoL using generic SF-36 and specific MiniRQLQ questionnaires significantly and similarly improved regardless of rhinitis aetiology.23 In contrast to SF-36, the improvement in MiniRQLQ was parallel with the symptoms in means of significance. This may be due to the fact that SF-36 is used to assess the general aspects of HRQoL and does not give specific information about rhinitis, whereas MiniRQLQ is discriminative and informative for the rhinitis.17,18
Since a variety of cytokines are associated with lower quality of sleep, intranasal corticosteroids which have high anti-inflammatory effect have been shown to improve sleep problems.15,33 Even though there was a significant improvement in snoring symptom in both rhinitis, sleep quality did not change as shown by ESS. This might be because our study group was composed not only with severe types, but it also included mild forms of rhinitis.
TANS was well tolerated in both groups, whereas the most seen adverse events were headache and bitter taste. The adverse events may be mitigated by the dosing technique recommended in the product labelling. The prevalence of other treatment-related adverse effects did not differ significantly between the groups, which was consistent with the literature.23
In summary, nasal triamcinolone was effective on nasal and extra-nasal symptoms, and reduced the HRQoL impairment in NAR, as much as in AR with no serious adverse events. Our study disproved the idea of ineffectiveness of corticosteroid treatment in NAR, and showed that triamcinolone acetate may be an alternative drug in the treatment of NAR. We may speculate that the effectiveness of intranasal triamcinolone in NAR may support the existence of a common inflammatory mechanism in NAR with AR. However, further investigations are required to state such conclusion.
Ethical disclosuresPatients’ data protectionConfidentiality of data. The authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentRight to privacy and informed consent. The authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Protection of human subjects and animals in researchProtection of human and animal subjects. The authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
FundingThis work was supported by a grant from Kirikkale University Projects of Scientific Researches (grant no: 2007/4) which was received by Baççıoğlu Kavut, and Kalpaklıoğlu.
Conflict of interestThe authors have no conflict of interests to declare.