INTRODUCTION
Pseudoephedrine is a direct and indirect acting sympathomimetic agent. It is a steroisomer of ephedrine and has similar actions. Pseudoephedrine and its salts are given orally for the symptomatic relief of nasal congestion. They are also commonly combined with other ingredients in preparations intended for the relief of cough and cold symptoms. Some cases of adverse cutaneous effects associated with pseudoephedrine have been reported (recurrent pseudo-scarlatina, fixed drug eruptions, erythematous macular rash, systemic contact dermatitis...). Considering the frequent use of pseudoephedrine, associated drug eruptions generally appear to be rare.
Phenylephrine is a sympathomimetic agent with mainly direct effects on a -adrenergic receptors. It has been used parenterally in the treatment of hypotensive states, but this drug and its salts are more commonly employed as local vasoconstrictors, either topically or by mouth for the symptomatic relief of nasal congestion, and in ophthalmology as a mydriatic or as a conjunctival decongestant (1). Since the first description of allergic contact dermatitis from phenylephrine in eye drops in 1979, further cases have been described; however, it seems to be a rare condition despite extensive use by ophthalmologists (2-21).
We report the case of a patient who had an allergic contact dermatitis due to phenylephrine in eye drops and some years later an erythrodermia after oral ingestion of pseudoephedrine. We have not found any case similar to this in the literature.
Case report
A 18-year-old woman was referred to our outpatient clinic because she had developed 6 months before pruritus with slight erythema in the trunk that became generalized until erythrodermia was finally produced. This reaction happened 3-4 hours after taking a tablet of Narine® (pseudoephedrine and loratadine). The patient was treated with systemic corticosteroids and antihistamines. She presented generalized skin desquamation and the complete resolution of the symptoms was in 2 weeks.
Four years before, she had suffered eyelid erythema, ocular itching and conjunctival hyperemia 24 hours after the application of 2 kinds of eye drops: Oculos Fenilefrina® 10 % (phenylephrine) and Colircusi Tropicamida® (tropicamide). The reaction resolved with topical corticosteroids within 5-7 days.
The patient had previously tolerated these drugs. After that, she had employed some eye drops with local anesthetics, ciclopentolate and atropine, and oral loratadine without problems.
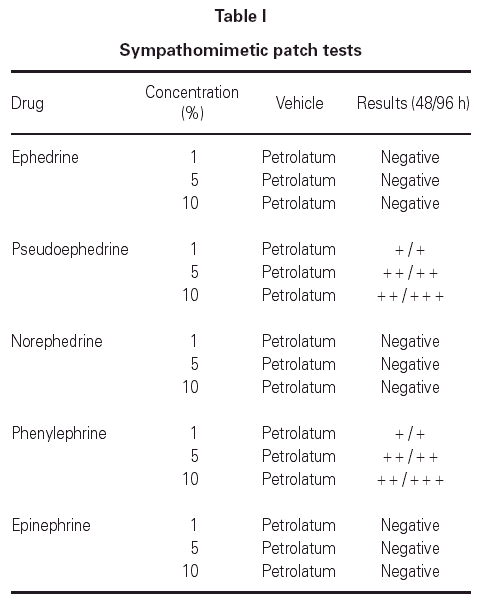
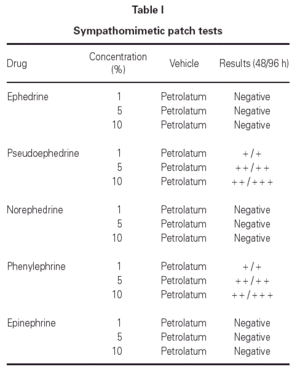
Patch tests with European standard series, Oculos Fenilefrina® 10 % and Colircusi Tropicamida® eye drops, pseudoephedrine, phenylephrine and other sympathomimetic agents were applied to the patient's back. The patch tests were removed after 48 hours, and readings were performed at 48 and 96 hours according to the recommendations of the ECDRG (European Contact Dermatitis Research Group). The European standard series and Colircusi Tropicamida® eye drop proved negative. After 48 hours the patches with Oculos Fenilefrina® 10 % eye drop, pseudoephedrine and phenylephrine were positive (table I).
Excipients in Oculos Fenilefrina® 10 % eye drop were not tested because we confirmed that they were included in other eye drops that the patient used without problems.
We informed our patient that she had to avoid both drugs, phenylephrine and pseudoephedrine, but 4 months later she consulted again with an erythrodermic reaction after having taken "one tablet" for cold symptoms. We confirmed that this tablet included pseudoephedrine. A cutaneous biopsy was carried out showing hydropic changes in the basal layer and, in the dermis, moderate edema with slight perivascular infiltrate of lymphocytes and eosinophils. The reaction resolved with oral antihistamines and corticosteroids within 7 days.
Discussion
We present a patient with 2 delayed cutaneous reactions from 2 sympathomimetic drugs: an allergic contact dermatitis due to phenylephrine and an erythrodermia due to pseudoephedrine. Although there are several reports of allergic contact dermatitis caused by phenylephrine (2-21), we have only found 2 cases of erythrodermia related with pseudoephedrine (22, 23) and no cases of both reactions in the same patient as in our case.
Both drugs belong to the phenylamine family, which comprises 2 main groups of drugs: phenylpropanolamin-derived (ephedrine, pseudoephedrine, and norephedrine) and phenylethanolamin-derived (phenylephrine and epinephrine) (1). The very close chemical structures of these drugs could explain potential crossreactions among them. Although some authors have observed cross-reactions between ephedrine and pseudoephedrine (24, 25), others have not (26). Interestingly, in some cases patch tests proved positive with pseudoephedrine and phenylephrine with not clinical relevance (25, 26). Audicana et al reported a case of generalized eczema caused by pseudoephedrine taken orally in a patient with previous allergic contact dermatitis by phenylephrine (14). In our case, the histology is similar to that described in the case of Vega et al (27) and ruled out the diagnosis of eczema. So, we think that our patient has presented 2 different reactions with different clinical evolution and histopathology, probably not due to cross-reactivity between the drugs incriminated. We have not found similar coincidence in the literature.