Hypersensitivity occurs when the body is stimulated by an antigen, resulting in an immune response, and leads to a physiological disorder or abnormal tissue trauma. Various immune cells, cytokines, and inflammatory mediators are involved in the immune responses related to allergic diseases, which are the core of anaphylaxis. Estrogen receptors are widely distributed in immune cells, which combine with estrogen and participate in allergic responses by affecting immune cells, cytokines, and inflammatory factors. We aimed to summarize the association between estrogen and allergic reactions to provide a scientific basis for understanding and studying the mechanisms of allergic diseases.
Disorders of environmental homeostasis in the barrier site caused by either internal and/or externally driven perturbations subvert tissue homeostasis can activate or reduce the threshold activation of the immune system, which is a major requirement for allergic sensitization.1 Hypersensitivity is usually mediated by biased Th cells and harmful IgE, and allergic rhinitis, allergic asthma, allergic dermatitis, anaphylactic shock, food and drug allergy are common clinical allergic diseases.2,3 Some clinical studies on gender differences in the incidence of systemic allergic reactions showed that women were more susceptible to allergic reactions and idiopathic allergic reactions caused by food, drugs, and radiocontrast agents than men,4,5 and these gender differences occurred in adulthood rather than at prepuberty.6,7 Women outnumber men in the number and severity of acute and chronic allergic reactions, and clinical reports show that allergic reactions occurring during the menstrual period are recurrent and cyclical.8–12 In addition, Hox et al. established a mouse model of anaphylaxis and found allergic reactions in female mice were estrogen-dependent, and compared with male mice, the severity and duration of allergic reactions in female mice significantly increased.13 These findings suggest that estrogen might be involved in gender differences in the prevalence of allergic diseases and participate in the susceptibility to allergic reactions.
Estrogen and estrogen receptor (ER) signaling involvement in the immune responses to allergic diseasesEstrogen is a G18 steroid hormone that is converted via androgenesis and catalyzed by aromatase, which plays an important role in the reproductive system. Estrogen also has important impacts on the immune system.14 There is a significant gender difference in the ability to induce an immune response. In clinical studies, the humoral and cellular immune responses of women were found to be stronger than those of men. Women were more prone to autoimmune diseases than men, and disease severity varied with the level of estradiol in women, especially during the menstrual cycle and pregnancy.15–17 The principal factor accounting for the difference in immunity between sexes is the difference in estrogen levels, suggesting that estrogen might be an important factor in allergic immunological responses.
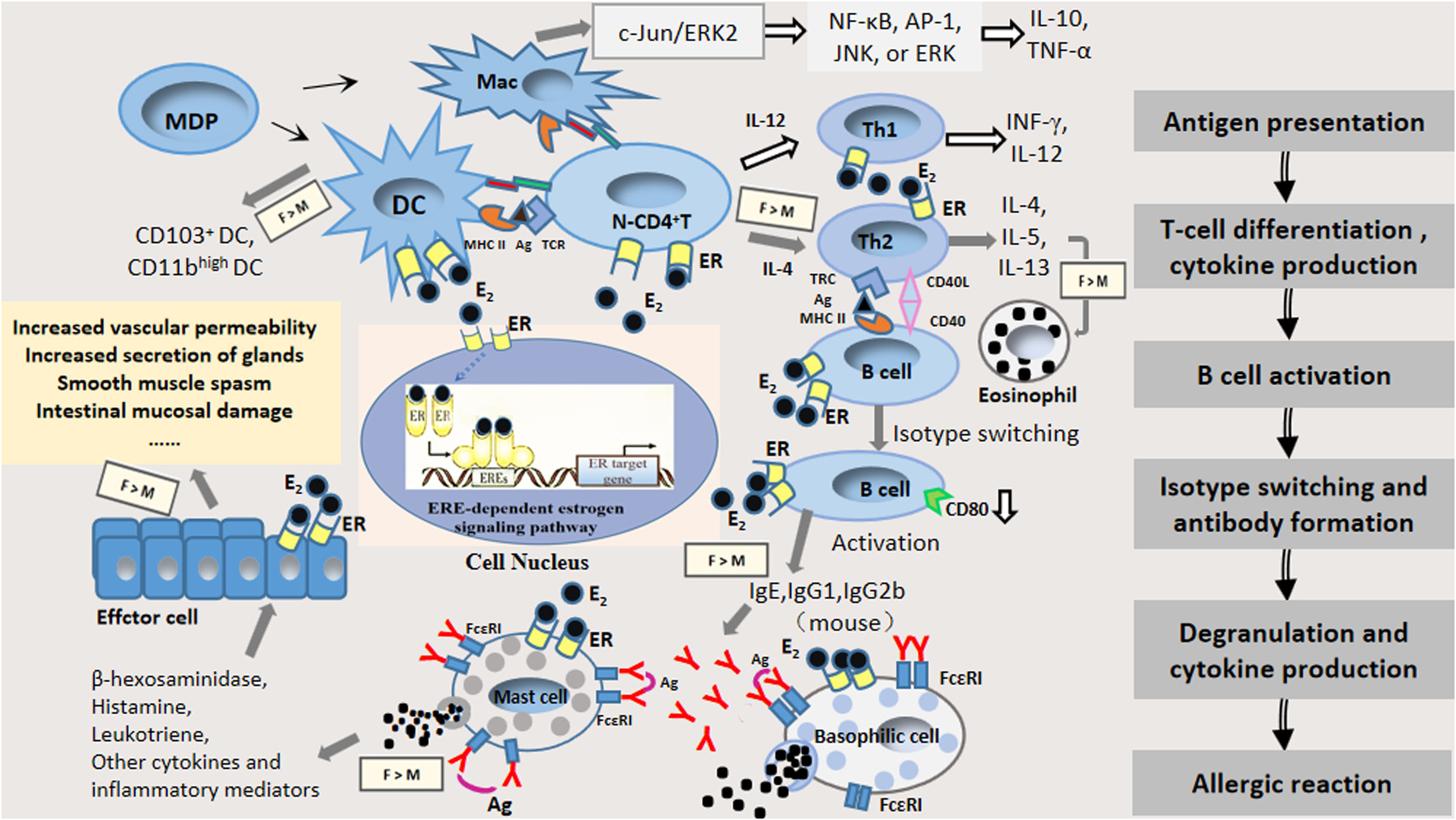
Estrogen plays its role mainly by binding with ER; ERs are mainly classified as ERα and ERβ, which are the products of different genes and possess 56% homology. Both ERs contain a DNA-binding domain and contain six regions, denominated as A through F, and are divided into four functional domains. A very strongly conserved domain is the C-terminal region, which separates to form highly variable NH2-terminal regions (A and B domains) containing the ligand-independent activation function 1 (AF-1), a COOH terminal interaction, a DNA-binding domain (D domain), a ligand binding domain (E domain), and an F domain. The estrogen response element, ERE, contains a nuclear localization signal sufficient to direct an autonomous activating domain in a ligand-dependent transactivation function (AF-2), and residues that are involved in an interaction with heat shock protein 90kDa (Hsp 90).18–21 Upon binding to estrogen, the ER undergoes a conformational change and then binds to its homologous DNA target site in the regulatory region of the estrogen-inducible gene (known as an estrogen response element, ERE), thereby regulating estrogen-responsive genes.18 ERs are widely distributed in immune cells22,14 so that estrogen modulates the proliferation and function of immune cells involved in allergic reactions, and stimulates the secretion of the corresponding cytokines that produce different biological effects, thus affecting the function of the immune system and playing an important role in every phase of allergic reactions.23–34Fig. 1 presents a flow diagram of immune development leading to allergic sensitization with known expression of estrogen receptors.
The pattern of estrogen and estrogen receptor involvement in the immune response of allergic diseases with known expression of estrogen receptors by each immune cell type: antigen presentation, Th2 polarization, isotype switching, mast cell degranulation and the effect stage. Abbreviations: E2: estrogen; ER: estrogen receptor α or estrogen receptor β; IgE: immunoglobulin E; IgG1: immunoglobulin G1; IgG2b: immunoglobulin G2b; Ag: antigen; MDP: macrophage and dendritic cell progenitor cell; DC: dendritic cell; Mac: macrophage; N-CD4+ T: native CD4+ T cell; Gray arrow represents enhancement of estrogen; White arrow represents inhibition of estrogen; F: female; M: male.
Antigen presentation is the initial stage of the immune response and is mainly a process in which antigen-presenting cells (e.g., dendritic cells (DCs), macrophages) take up and process them into antigenic peptides so that they can be recognized by immunocompetent cells.22 Antigenic peptides bind to major histocompatibility complex II (MHCII) molecules on the surface of antigen-presenting cells and are further transported by antigen-presenting cells to the Pies lymph node and mesenteric lymph node, where they stimulate native T cells toward distinct effector T-cell subsets (e.g., Th1, Th2, Th17), or induce tolerance through the induction of regulatory T cells and activate a subsequent immune response.2,35 Estrogen affects the expression of cytokines and antigen presentation in DCs and macrophages. The binding of estrogen to its receptors on antigen-presenting cell membranes, such as on DCs and macrophages, could regulate their polarization and function and affect antigen phagocytosis and presentation.36,37 Thus, the effects of estrogen and ERs on the functions of antigen-presenting cells, and the direct effect of ER signaling on other immune cell types might influence the progression of allergic diseases.
DCs are the most powerful antigen-presenting cells, which are the initiators of specific immune responses, and ongoing investigations revealed that asthma and allergies are closely related to the CD4+ Th2 type response caused by allergens. This effect was inseparable from the related signaling pathways initiated by mucosal epithelial DCs.38 Estrogen inhibited DCs to produce IL-12, TNF-α, and IFN-γ in young adult rats, whereas in older rats, the effect is reversed. These findings might be related to the different levels of ERα and the varying levels of differentiation of DCs in rats at different ages.39 The migration of bone marrow DCs and plasmacytoid DCs to pulmonary draining lymph nodes increased in ovalbumin (OVA)-sensitized and challenged female mice compared to male BALB/c mice.40 Masuda et al. found the number of CD11bhigh DCs and CD103+ DCs in the lung and bronchial lymph node (BLN) was increased to a greater extent in female mice than in male mice after OVA inhalation. They also found 17β-oestradiol enhanced CD86 expression on CD103+ DCs after allergen exposure in vitro.37 Some researchers have suggested that ERα signaling may influence two different mechanisms of DC-mediated immune responses during inflammation in vivo, namely, (1) regulating the production of inflammatory DCs in draining lymph nodes or inflamed tissues and development, which is crucial for the adaptive immune response; (2) regulation of pro-inflammatory and T-cell stimulatory responses of mature DCs.41 The role of ER ligands depends on the extracellular cytokine environment. The same highly purified cell types can respond differently to estradiol or ER antagonists. The ER signal in DC progenitor cells regulates cell differentiation signals through differential interactions with cytokine receptors.
Macrophages are another important class of antigen-presenting cells and one of the most active types of cells in the body. Macrophages are involved in the immune response via induction of oral intolerance or allergic sensitization.42 In addition, macrophages express T-cell immunoglobulin and the mucin domain (Tim) gene family, which have been shown to be involved in asthma, allergic rhinitis, food allergy, and autoimmunity.43 In OVA-sensitized and challenged female mice, the percentage of alveolar macrophages increased compared to male BALB/c mice.40 Carreras et al. found that E2 and ERα signaling led to dendritic cell functional polarization through increased expression of the interferon regulatory factor-4 transcription factor, which is also involved in alternative activation of macrophages in C57BL/6 mice.44 Estrogen tends to stimulate macrophage migration. Moreover, estrogen significantly decreased ERα- or ERβ-mediated IL-10 and TNF-α production by macrophages alongside the modulation of NF-κB, AP-1, JNK, or ERK signaling pathways in human M1 and M2 macrophages.45 These mediators are secreted by macrophages as a result of the activation of the c-Jun/ERK2 complex, which could cause the inhibition of transcription of the ER gene. The estrogen/ER pathway regulates the phenotype and function of human macrophages; thus, ER signaling might represent a possible pharmacological target in treating inflammatory diseases.46,47
Estrogen promotes T-cell differentiation and affects the function of T cellsAntigen-presenting cells activate primary CD4+ T cells through a series of signaling pathways and cause T cells to differentiate into Th2 cells for further proliferation. Th2 cells secrete a large number of Th2 cytokines, such as Interleukin-4 (IL-4), Interleukin-5 (IL-5), Interleukin-13 (IL-13), which can selectively induce the conversion of B cells into plasma cells, producing antigen-specific IgE antibodies that put the body in a sensitized state.24,25,48–51 Thus, Th2 cells are the major Th cell subset involved in the hypersensitivity sensitization phase.23,52–54 Allergen-specific Th2 cells are essential for the production of Th2 cytokines and Th2 cytokine-recruited effector cells, and initiate Ig class-switching to the IgE isotype in B cells.55
The immune status of Th1/Th2 is currently an ideal index to evaluate immune function, and the study of the role of estrogen in regulating the balance of Th1/Th2 has garnered increased attention. One study found that in the peripheral blood of patients with premature ovarian failure, the ratio of Thl/Th2 cells was significantly higher than that in the control group mice.56 In vitro stimulation of estrogen increases the secretion of IL-10 and down-regulates the secretion of TNF-α by the same antigen-activated T cells, e.g., the shift in T-cell response to Th2, suggesting that estrogen regulates two types of cytokines: estrogen inhibits the production of Th1 cytokines which have an inhibitory effect on Th1 cells, while estrogen stimulates the production of Th2 cytokines that improve Th2 cell growth and proliferation.57 CD4+ T cells cultured with CD103+ DCs from female mice produced higher levels of IL-4, IL-5, and IL-13 compared with male mice. Furthermore, the estradiol induced the enhanced IL-5 production from CD4+ T cells in vitro.37 Hormone upregulation of the Th2 response might further exacerbate the susceptibility of atopic diseases. A human study showed that when estrogen acted on the immune system, it affected the body's immune function by regulating the dynamic balance between Th1/Th2.58 At the same time, we should pay attention to the effects of estrogen and other immune factors related to allergies and Th2 disorders, including the balance of cytokines produced by Th1 and regulatory T cells, and the inflammatory cytokine effects in the Th17 pathway driven by allergy.59
As one of the most important adaptive immune response cells, T lymphocytes play an important role in cellular immunity and humoral immune regulation. The reason for estrogen's immunoregulatory and protective effects may be that estrogen regulates T-cell immune responses in a concentration-dependent manner. The immunoregulatory effect of estrogen on T cells depends on its concentration and its receptor subtypes. Both nuclear and membrane-associated ERs regulate the mechanism of T lymphocytes in different ways, and the multiple effects on Th immunity also vary with the types of immune cells or animal model utilized.
Estrogen alters the phenotype and function of B cellsThe transcriptional regulation mediated by ER induces the class transformation and recombination of the immunoglobulin heavy chain variable region (VH) to the DH-JH gene and somatic super mutations in developing B cells.60 One study indicated that estrogen promoted the activity of B cells through the down-regulation of CD80 expression on B cells.61 Estrogen enhances the activity of peripheral B cells by an increased number of Ig-producing cells as well as an increased Ig production in estrogen-treated mice.62 Furthermore, ER signal transduction plays a protective role in immunization by regulating B-cell activation, which is significant in determining the pathogenesis of autoimmune diseases and cancer.60 B cells are not only professional cells that produce antibodies, but also secrete many cytokines that relate to inflammation and have the ability to present antigens, which are closely related to allergic diseases.63,64
Specific identification of these epitopes on the allergen by combining IgE antibodies with effector cells is essential for the development of allergic diseases.65 The primary B cells that have not encountered the antigen express immunoglobulin M and immunoglobulin D on their surface. During an immune response, B cell clones to produce IgG, IgA, or IgE, IgM, or IgD antibodies through antibody isotype-switching.66 Specific IgG antibodies play a crucial role in the pathogenesis of allergic diseases. One study showed that estrogen treatment increased antibody subclass IgG1 and IgG2b levels in female mice.67 IgE is an important marker of allergies which can regulate allergic reactions while playing an immunomodulatory role in allergic rhinitis and asthma.38 Sakai et al. found phytoestrogen enhanced the production of allergen-specific IgE from splenocytes in BALB/c mice.68 Furthermore, excessive secretion of Th2 cytokines (IL4, IL-5, IL-13) led to an increase in allergen-specific antibody IgE antibodies and infiltration of eosinophils.69
In the process of antibody production, suppressor T cells can inhibit the maturation of B cells and the production of antibodies,70 while helper T cells facilitate B cells maturation and the production of antibodies.25,48–51 Therefore, estrogen might affect the maturation of B cells and the production and secretion of antibodies via effects on development and maturation of suppressor T cells and helper T cells in vivo under certain conditions. In conclusion, the effects of estrogen on the sensitization phase of allergic diseases appear to be crucial and extremely complex. Therefore, there is no doubt that new ideas could be explored for research of allergic diseases if their etiology and those of other immune disorders are explored from the perspective of ER signaling.
Effects of estrogen on the provocation and effect phase of allergic reactionsMast cells are known as immediate hypersensitivity effector cells, and are widely found in barrier tissues associated with the external environment such as the skin or the mucosa of the respiratory and gastrointestinal tract.71 Mast cells are also degranulated in response to stimulation by the FcɛRI-mediated pathway, induced by the Ca2+ ionophore and compound 48/80. The release of major mediators of immediate hypersensitivity (e.g., histamine, cysteinyl leukotrienes) is an essential process in allergic reactions.72 In vitro studies have found that the combination of physiological concentrations of estradiol or environmental estrogen and membrane ERs trigger a rapid influx of extracellular Ca2+, which results in the release of pre-formed granule protein beta-hexosaminidase, and the synthesis and release of leukotriene C4, as well as enhancing the IgE-dependent release of these media.34 Estradiol enhances IgE-dependent activation, IgE-induced degranulation, and LTC4 production of the rat and mouse mast cell, resulting in a shift in allergen dose-responses.73–75 Studies have suggested that estrogen and estrogen-like compounds combine with ERs to stimulate calcium signaling, and, thus, induce mast cell degranulation.73,76 Compared with male mice, the IgE level in the serum of OVA-sensitized and challenged female mice increased.77,78 Estrogenic environmental pollutants induce rapid β-hexosaminidase dose-dependent release from mast cells, further strengthening the evidence that estrogen enhances IgE-mediated release in mast cells.79 In addition, estrogen treatment of peritoneal mast cells stimulated by IgE in female rats increased the release of histamine, which was decreased by progesterone, testosterone, or 5α-DHT. Therefore, sex hormones could regulate the activation of mast cells.80,81 The catalytic role of estrogen in the growth of endometriosis lesions, and the mechanism involved in estrogen-mediated endometriosis in rats may be related to the activation of mast cell degranulation by estrogen. Elevated E2 concentration might be a key factor in the degranulation and recruitment of mast cells in mouse with ovarian endometriosis.81,82 However, another study showed that the effect of estradiol on allergic reactions was independent of changes in mast cell reactivity, suggesting that estrogen-mediated allergic reactions were enhanced after activation of mast cells and unrelated to the trigger mechanism.13 In addition, estradiol was also found to be ineffective in altering the degranulation of human basophils. However, studies have also reported that IgE/Ag-induced degranulation was enhanced approximately 4% in transformed mast cells (RBL-2H3 and HMC-1).83
With regard to the effect of estrogen on the effect phase of allergic reactions, research has confirmed that in IgE-mediated passive systemic hypersensitivity (PSA), the body temperature drops more obviously in female mice compared with male mice. As estrogen regulates the expression of eNOS and the production of NO, the vascular permeability of female mice is significantly increased. The edema around the bronchus was found to be more extensive, and rich red blood cells were more abundant in female mice as confirmed by lung histology, which could enhance allergic reactions.13 Clinical reports also indicate that “female strength” existed not only in IgE-mediated allergic reactions but also in allergic reactions induced by drugs and radioactive compounds that did not involve FcɛRI.6,84
At present, few studies have focused on the effects of estrogen during the provocation and effect phases of allergic reactions, and many controversies remain. The specific mechanism requires further laboratory and clinical research to promote an understanding of the etiology and pathogenesis of allergic diseases. In addition, the specific mechanism of estrogen's effect on mast cell response from different gender sources and the gender-dependent effect of estrogen on signaling pathways in MC cells should be considered in relevant studies. In animal models of disease, the use of male animals may neglect critical interactions between specific genes or mutations and dominant pathways in females, such as the NO pathway, which may be significantly associated with gender-related allergic disease risk.
Concluding remarksOne way to provide insights into human diseases is to identify physiological factors that regulate the function of immune cells. ERs are found in many immunoregulatory cells, and estrogen levels or other ER ligands significantly affect the development and function of immune cells. Therefore, estrogen and ERs play an extensive role in allergic diseases. Estrogen causes an immune response deviation, which is conducive to promoting Th2 polarization-induced allergic reactions, the transformation of IgE-producing B cells, as well as promoting the degranulation of mast cells and basophils. The intensity of the ER signal can reflect gender differences and the effect of pregnancy on autoimmunity and infection. There are numerous immune cells, cytokines, and inflammatory mediators involved in various stages of immune response to allergic diseases. As an important immune regulatory factor, estrogen is undoubtedly involved in the regulation of many cells, cytokines, and inflammatory mediators which are the core elements of allergic reactions.
However, the role of estrogen in allergic diseases remains complex. As the prevalence of allergic disease, and, particularly, the impact on women continue to increase, it is essential to comprehend the effect of estrogen on these diseases. With the gradual expansion of research regarding the relationship between gene polymorphisms of estrogen and its receptor, signal pathways, estrogen membrane surface-associated receptors, and immune responses in allergic diseases, further clarification of the pathogenesis of allergic diseases and other immune diseases will provide new methods and a basis for the treatment of allergic diseases.
FundingThis paper was supported by Genetically Modified Organisms Breeding Major Projects (2016ZX08011006), which did not play any role in writing the manuscript, or the decision to submit the manuscript for publication.
Conflict of interestThe authors have no conflict of interest to declare.
We thank all colleagues who contributed to this paper. ZY and HL prepared the first draft of the manuscript. S and CC helped to draft the manuscript. All authors read and approved the final version. We would like to thank Tsinghua University (2016ZX08011006) for publication support.