Angiotensin converting enzyme (ACE) is expressed at high levels in the lungs and plays a role in the metabolism of the endogenous peptides involved in asthma pathogenesis. ACE gene polymorphisms have been reported to be linked to asthma. However, the results are conflicting, with no reported studies on Egyptian asthmatics. We aimed to assess ACE gene polymorphism among Egyptian asthmatics, and to determine its possible association with asthma severity.
MethodsThis case–control study was conducted on 30 adult asthmatic patients, and 30 healthy controls with no history of asthma or atopy. Atopic status among asthmatics was determined by skin prick test (SPT). Lung functions were assessed by spirometry. Determination of ACE genotypes was performed for all subjects. Total serum IgE levels were measured by ELISA.
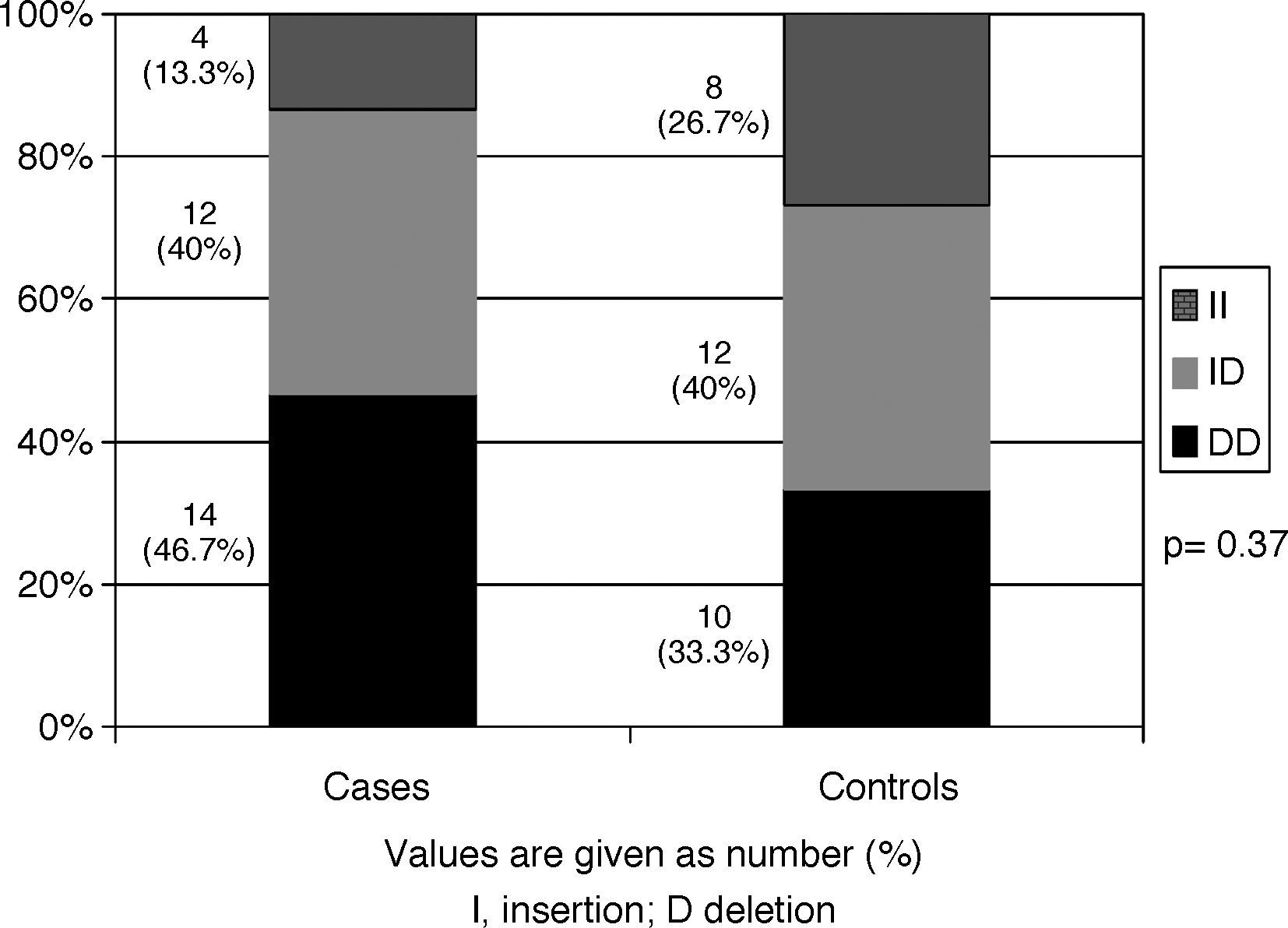
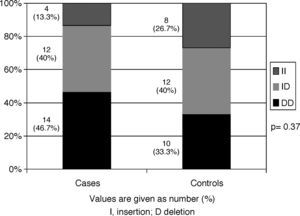
ResultsThe frequencies of the DD, ID and II genotypes were 46.7%, 40%, and 13.3%, respectively among the cases, and 33.3%, 40%, and 26.7%, respectively among the controls. No significant differences in ACE genotype distribution were observed between cases and controls (p=0.37). Genotype distribution did not differ according to age of onset or severity of asthma, total serum IgE levels, SPT positivity, or number of positive SPT reactions. Furthermore, ACE polymorphism was not statistically different between asthmatic patients without any associated atopic disease and those with an associated atopic disease.
ConclusionThe results of our study indicate that ACE gene polymorphism is not significantly associated with bronchial asthma or with its severity among Egyptian adults.
Asthma is broadly defined as a chronic disease of the airways that is characterised by variable airflow obstruction, bronchial hyper-responsiveness, and underlying inflammation.1 Asthma is a complex process that depends on the interplay between genetic factors and environmental exposures.2 It is impossible to relate one single gene, or a group of genes to asthma. Numerous studies have examined polymorphisms in several hundred genes for association with asthma and allergy phenotypes.3,4
Angiotensin converting enzyme (ACE) gene is one of these genes. ACE is expressed at high levels in the lungs, and exhibits an anti-inflammatory effect through catabolising bradykinins and tachykinins, which induce smooth muscle airway constriction, bronchial edema, mucus hyper-secretion, and neurogenic inflammation.5 On the other hand, ACE converts angiotensin I into the vasoactive angiotensin II, which through causing proliferation and contractility of airway smooth muscles, can contribute to excessive airway obstruction.6
The ACE gene contains a polymorphism based on the presence (or insertion [I]), or absence (or deletion [D]) of a 287-base pair element in intron 16 on chromosome 17q23. According to the presence of this element, three different genotypes may occur: DD and II homozygotes, and ID heterozygotes.7 Several studies have investigated the association between ACE gene polymorphism and asthma. There are however many inconsistencies in these studies; whereas some studies have shown a significant association between DD polymorphism and asthma,8–10 others have reported no significant association.11–14 This discrepancy has been attributed to the ethnic diversities in the various studies.
We therefore aimed to assess ACE gene polymorphism among Egyptian asthmatics, a previously unstudied ethnic group, and to determine its possible association with asthma severity.
Materials and methodsThis case–control study was conducted on 30 adult asthmatic patients who were recruited from the Allergy and Clinical Immunology outpatient clinic of Ain Shams University Hospital over a six-month period from December 2009 to May 2010. Asthma was diagnosed according to the guidelines of the Global Initiative for Asthma (GINA) 2007.15 The study also included a control group of 30 healthy individuals with no history of asthma, atopy or other allergic diseases, and with normal spirometric results. Subjects with cardiac diseases or hypertension were excluded due to the possible association of these diseases with ACE gene polymorphisms.16,17 An informed consent was obtained from all participants, and the study was approved by the Research Ethics Committee of Ain Shams University.
All cases completed a questionnaire on asthma symptoms, triggering factors and other associated atopic diseases. Atopic status among cases was determined by skin prick test (SPT) reaction to the most common locally encountered allergens. These included mites, mixed pollens, moulds, cat hair, dog hair, horse hair, pigeon, cockroach, and tobacco. Histamine (0.1%) in phosphate-buffered saline and physiologic saline were used as positive and negative controls, respectively. After 20min, a wheal size ≥3mm greater than the negative control was considered a positive result.
Spirometry was performed at the Pulmonary Functions Laboratory at Ain Shams University Hospital, using the Flowmate V Plus spirometer (Spirometrics, ME, USA). Reference values used were those proposed by the European Respiratory Society.18
Determination of ACE genotypesPeripheral blood samples were obtained from all individuals. Genomic DNA was extracted using phenol–chloroform and stored at −20°C. The ACE gene I/D polymorphism was determined by real-time polymerase chain reaction using MagNA Pure LC Total Nucleic Acid Isolation Kit (Roche Diagnostics, Mannheim, Germany), and LightCycler FastStart DNA MasterPLUS SYBR Green I kit (Roche Diagnostics, Mannheim, Germany). The sense oligonucleotide primer was 5′-CTG GAG ACC ACT CCC ATC CTT TCT-3′, and the antisense primer was 5′-GAT GTG GCC ATC ACA TTC GTC AGA T-3′. The reactions were performed according to the manufacturers’ instructions. Subjects were classified into the DD, ID, or II genotype according to the presence (or insertion [I]), or absence (or deletion [D]) of the 287-base pair insertion in intron 16 of the ACE gene.
In addition, total serum IgE levels were measured using a sandwich enzyme-linked immunosorbent assay (ELISA) kit (BioCheck Inc., CA, USA). The normal limit of total IgE was 100IU/mL.
The primary outcome measure of the study was the difference in frequency of ACE gene polymorphisms between asthmatic patients compared to healthy controls. Secondary outcome measures were possible associations of ACE gene polymorphisms with asthma severity, atopic status, and with the different associated atopic diseases.
Statistical analysisData were analysed using SPSS (Statistical Program for Social Sciences) version 12. Data were expressed as mean±standard deviation (SD) for parametric data, and as median and interquartile range (IQR) for non-parametric data, respectively. Parametric data were analysed using Student's t-test and one-way analysis of variance (ANOVA) for the comparison of two and three groups, respectively. Non-parametric data were analysed using the Mann–Whitney U and Kruskal–Wallis tests to compare two and three groups, respectively. Chi-square test was used to compare categorical data. A p-value of less than 0.05 was considered significant.
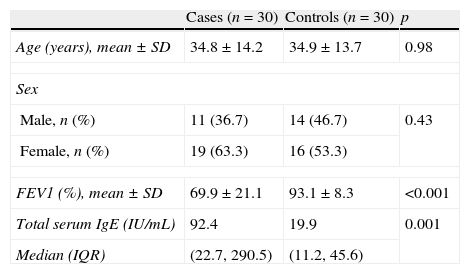
ResultsThis case–control study was conducted on 30 adult asthmatic patients and 30 healthy controls. As shown in Table 1, both groups were comparable for age and sex. The mean percentage of predicted forced expiratory volume in 1s (FEV1) was significantly lower among cases than among controls. Asthmatic patients had significantly higher levels of total serum IgE than controls.
Characteristics of the study groups.
| Cases (n=30) | Controls (n=30) | p | |
| Age (years), mean±SD | 34.8±14.2 | 34.9±13.7 | 0.98 |
| Sex | |||
| Male, n (%) | 11 (36.7) | 14 (46.7) | 0.43 |
| Female, n (%) | 19 (63.3) | 16 (53.3) | |
| FEV1 (%), mean±SD | 69.9±21.1 | 93.1±8.3 | <0.001 |
| Total serum IgE (IU/mL) | 92.4 | 19.9 | 0.001 |
| Median (IQR) | (22.7, 290.5) | (11.2, 45.6) | |
FEV1, forced expiratory volume in 1s; IgE, immunoglobulin E; IQR, interquartile range.
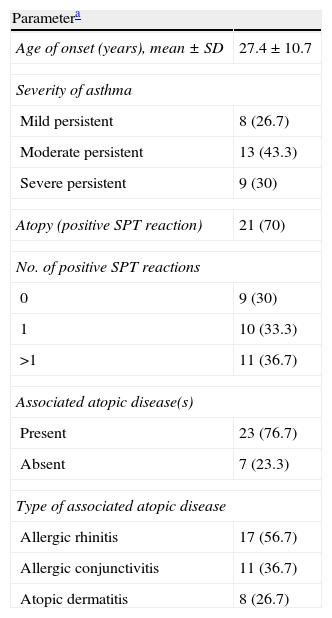
The characteristics of asthmatic patients are summarised in Table 2. The majority of patients (43.3%) had moderate persistent asthma. More than two thirds of the patients (70%) were atopic, having a positive SPT reaction to the tested allergens, and more than 75% had an associated atopic disease. The most frequent associated atopic disease observed was allergic rhinitis (56.7%), followed by allergic conjunctivitis (36.7%), and atopic dermatitis (26.7%).
Characteristics of the asthmatic patients.
| Parametera | |
| Age of onset (years), mean±SD | 27.4±10.7 |
| Severity of asthma | |
| Mild persistent | 8 (26.7) |
| Moderate persistent | 13 (43.3) |
| Severe persistent | 9 (30) |
| Atopy (positive SPT reaction) | 21 (70) |
| No. of positive SPT reactions | |
| 0 | 9 (30) |
| 1 | 10 (33.3) |
| >1 | 11 (36.7) |
| Associated atopic disease(s) | |
| Present | 23 (76.7) |
| Absent | 7 (23.3) |
| Type of associated atopic disease | |
| Allergic rhinitis | 17 (56.7) |
| Allergic conjunctivitis | 11 (36.7) |
| Atopic dermatitis | 8 (26.7) |
SPT, skin prick test.
ACE genotype distributions are shown in Fig. 1. In the asthmatic group, the frequencies of the DD, ID and II genotypes were 46.7%, 40%, and 13.3%, respectively. In the control group, the corresponding frequencies were 33.3%, 40%, and 26.7%, respectively. The DD genotype was more prevalent among asthmatics than among controls, yet this association did not achieve any statistical significance (p=0.37).
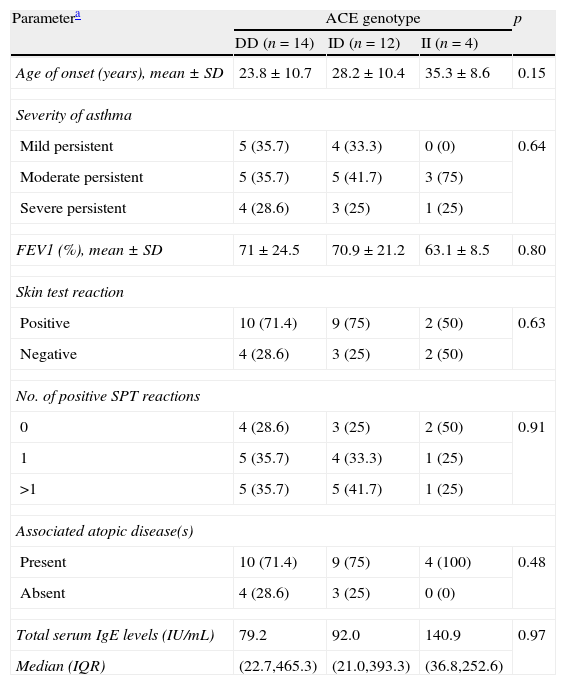
ACE genotypes in asthmatics according to the studied parametersACE genotype distributions according to the various studied parameters are shown in Table 3. There were no significant associations between genotype distribution and age of onset of asthma, FEV1 values, or total serum IgE levels. Genotype distribution did not differ significantly according to the severity of asthma, SPT positivity, or number of positive SPT reactions. Furthermore, ACE polymorphism was not statistically different between asthmatic patients without any associated atopic disease and those with an associated atopic disease.
ACE genotype distribution in asthmatics according to various parameters.
| Parametera | ACE genotype | p | ||
| DD (n=14) | ID (n=12) | II (n=4) | ||
| Age of onset (years), mean±SD | 23.8±10.7 | 28.2±10.4 | 35.3±8.6 | 0.15 |
| Severity of asthma | ||||
| Mild persistent | 5 (35.7) | 4 (33.3) | 0 (0) | 0.64 |
| Moderate persistent | 5 (35.7) | 5 (41.7) | 3 (75) | |
| Severe persistent | 4 (28.6) | 3 (25) | 1 (25) | |
| FEV1 (%), mean±SD | 71±24.5 | 70.9±21.2 | 63.1±8.5 | 0.80 |
| Skin test reaction | ||||
| Positive | 10 (71.4) | 9 (75) | 2 (50) | 0.63 |
| Negative | 4 (28.6) | 3 (25) | 2 (50) | |
| No. of positive SPT reactions | ||||
| 0 | 4 (28.6) | 3 (25) | 2 (50) | 0.91 |
| 1 | 5 (35.7) | 4 (33.3) | 1 (25) | |
| >1 | 5 (35.7) | 5 (41.7) | 1 (25) | |
| Associated atopic disease(s) | ||||
| Present | 10 (71.4) | 9 (75) | 4 (100) | 0.48 |
| Absent | 4 (28.6) | 3 (25) | 0 (0) | |
| Total serum IgE levels (IU/mL) | 79.2 | 92.0 | 140.9 | 0.97 |
| Median (IQR) | (22.7,465.3) | (21.0,393.3) | (36.8,252.6) | |
ACE, angiotensin converting enzyme; FEV1, forced expiratory volume in 1s; IgE, immunoglobulin E; IQR, interquartile range; SPT, skin prick test.
Bronchial asthma is a multifactorial disease influenced by genetic, environmental and neurohormonal factors. However, the nature of the genetic factors is unclear.19 Polymorphisms of the ACE gene have been shown to be associated with differences in plasma ACE levels. The insertion appears to reduce ACE expression. Thus, the DD genotype is associated with the highest plasma levels of ACE, while the II genotype is associated with the lowest levels.7 Several studies have attempted to find associations between ACE polymorphisms and the risk of asthma; however, the results have been very conflicting. To our knowledge, our study is the first to investigate the potential associations between polymorphisms in ACE gene and asthma among Egyptian asthmatics.
In the present study, we measured the frequency of ACE gene polymorphisms among 30 Egyptian adult asthmatics and 30 healthy controls. We found no significant differences between polymorphisms in the ACE gene between asthmatic patients and controls. A number of investigators have reported similar results. Chagani et al.20 found no significant association between ACE genotype distributions and asthma among Canadian asthmatics. Studies of the same polymorphism in Japanese,12,21,22 Korean,11,14 British,21 and Turkish23 adult asthmatics also reported similar distributions of ACE gene polymorphisms between asthmatic patients and controls. In striking contrast to the previous studies, fewer investigators demonstrated that ACE gene polymorphism was significantly associated with bronchial asthma. Benessiano et al.8 were the first to report the higher prevalence of the DD genotype among French asthmatic adults as compared to healthy controls. This was followed by similar studies among Czech24 and Turkish10 adults, and Taiwanese9 and Chinese25 children, confirming the association of ACE gene polymorphisms with an increased prevalence of the DD genotype in asthmatics. In our present study, we also detected a higher prevalence of DD genotype among asthmatics as compared to controls, although this association did not reach any statistical significance, perhaps due to the relatively small sample size of the study. The discrepancy between the findings of the previous studies may be explained by the ethnic differences between these studies.
The role of ACE genotypes in modulating the therapeutic response to ACE inhibitors has been demonstrated among patients with coronary heart disease,26 hypertension,27 diabetic nephropathy,28 and among patients who have undergone renal transplantation.29 Systemic reviews on these studies demonstrated a trend towards a better response in Caucasian patients with the DD genotype compared to those with the II genotype. However, among Asians, DD genotype patients did not benefit from ACE inhibitor therapy while II genotype patients showed large treatment responses.30,31 The difference in treatment responses between Caucasians and Asians demonstrates that ethnic differences affect these genotype–phenotype relationships.
Increased serum ACE levels may affect asthma severity, and studies have shown increased levels of angiotensin II in patients with severe asthma.32 In the present study, no association was observed between ACE gene polymorphisms and the severity of asthma, and the frequencies of the different genotypes did not differ according to the severity of asthma. Although the French study had shown an increased frequency of the ACE DD genotypes among asthmatic patients, no correlation could be demonstrated between disease severity and the increased frequency of this genotype.8 Similar findings have also been confirmed in other studies, demonstrating that ACE gene polymorphisms do not appear to be linked to the severity of disease among asthmatics.10–12,20,23
Several investigators have failed to detect any significant differences between ACE genotype distributions and atopy.10,12,21,33 A study on atopic Taiwanese children with allergic rhinitis demonstrated a significantly higher frequency of the DD genotype among patients with associated asthma than among those with allergic rhinitis only, and that allergic rhinitis patients with the DD genotype were over three times more likely to have associated asthma than those with the ID or II genotypes.9 Concerning the atopic status in our study, we failed to find any significant differences in the frequency of ACE genotypes between atopic and non-atopic asthmatics, and between asthmatic patients without any associated atopic disease and those with an associated atopic disease. ACE genotypes did not differ according to the number of positive SPT reactions. We also did not detect any significant association between ACE genotype distribution and total serum IgE levels, a finding similarly reported before.12,13 It is therefore probable that ACE gene polymorphism is associated more with asthma than with other atopic diseases.
In summary, although a slightly higher prevalence of the ACE DD genotype was detected among our asthmatic patients as compared to controls, the difference did not reach any statistical significance. Accordingly, our present findings cannot support the hypothesis that ACE gene polymorphisms are linked to asthma or to its severity among Egyptians. Further studies with a much larger patient population will be necessary to confirm this association. Discovery of an association of the ACE genotype with asthma among Egyptians might help to provide other options in asthma treatment.
Conflict of interestThe authors declare that no funding or grant was received for the study, and that they have no conflict of interest, financial or personal relationship related to the study.
The authors wish to thank Dr. Engy Ebeid Abd El-Meseh, M.B.B.Ch, for her great role in patient recruitment and data collection, and Dr. Mohamed Sharaf, director of the Finan Genetic Research Center, Cairo, for performing the laboratory analysis.