Allergic respiratory diseases are major health problems in paediatric population due their high level of prevalence and chronicity, and to their relevance in the costs and quality of life. One of the most important risk factors for the development of airway diseases in children and adolescents is atopy. The mainstays for the treatment of these diseases are avoiding allergens, controlling symptoms, and preventing them through sustained desensitization by allergen immunotherapy (AIT). AIT is a treatment option that consists in the administration of increasing amounts of allergens to modify the biological response to them, inducing long-term tolerance even after treatment has ended. This treatment approach has shown to decrease symptoms and improve quality of life, becoming cost effective for a large number of patients. In addition, it is considered the only treatment that can influence the natural course of the disease by targeting the cause of the allergic inflammatory response. The aim of this publication is to reflect the advances of AIT in the diagnosis and treatment of allergic respiratory diseases in children and adolescents reviewing articles published since 2000, establishing evidence categories to support the strength of the recommendations based on evidence. The first part of the article covers the prerequisite issues to understand how AIT is effective, such as the correct etiologic and clinical diagnosis of allergic respiratory diseases. Following this, the article outlines the advancements in understanding the mechanisms by which AIT achieve immune tolerance to allergens. Administration routes, treatment regimens, dose and duration, efficacy, safety, and factors associated with adherence are also reviewed. Finally, the article reviews future advances in the research of AIT.
Allergic respiratory diseases are a major health problem in the paediatric population due to their high level of prevalence and chronicity, as well as to their relevance in both the cost of healthcare and the quality of life of the patients and their families. According to large population-based studies, the prevalence of asthma and allergic rhinitis (AR) in children has increased in recent decades, presenting wheezing in the past year in up to 12% of children aged 6–7 years and to 14% at 13–14 years; AR has a prevalence of 15%.1–3 The total costs of asthma are greater in children than in adults due to the greater severity of the disease in this population,4 decreasing their quality of life in terms of health.5 Children with AR are twice as likely to suffer limitations in their activities, interfering with school performance in 40% with a nearly 30% decrease in productivity when their symptoms worsen.6
Findings have shown that atopy is among the most important risk factors for the development of airway diseases in children and adolescents.7–10 Exposure to allergens to which the child is sensitized to affects asthma control and severity, the prevalence of asthma morbidity in the form of asthma symptoms, hospitalizations and asthma mortality.11–14 The correlation between the onset of symptoms of asthma and AR has been proven,15 with the prevalence of asthma being three times higher in those who had AR and were atopic than if they were not atopic (39% versus 13%).6 Considering these chronic inflammatory diseases of atopic aetiology, the mainstays of treatment are avoiding allergens and other triggers, controlling symptoms and preventing them through sustained desensitization. The immune tolerance is achieved by allergen immunotherapy (AIT).
Although there is a consensus on carrying out allergen avoidance measures to prevent the occurrence of allergic respiratory symptoms, the prevention of exacerbations and control of AR by avoidance measures have provided discordant results.16 There is no evidence that supports a possible secondary prevention for the development of asthma in patients with AR. This, in part, may be explained since in atopic asthmatic children, small levels of common allergens found at home pose a significant risk of increased morbidity.17 Moreover, in real life the level of exposure to allergens remains high, even in those children who reported symptoms during these exposures.18
AIT is a treatment option used to achieve immune tolerance to clinically relevant allergens. The allergens are administered by increasing the dose modifying the biological response to decreased symptoms, improving quality of life, inducing long-term tolerance even after treatment and becoming cost effective for a large number of patients.19,20 Therefore, this treatment approach is considered the only one that can influence the natural course of the disease by targeting the cause of the allergic inflammatory response from its aetiology and, at the same time, benefiting different target organs in the same patient.
In paediatric patients, evidence is available on their effectiveness,21 on its prolonged effect after treatment22,23 and on its ability to prevent the development of asthma24,25 and new sensitizations.26,27 This protective and modifying effect of the evolution of AIT is advisable in the early stages of allergic respiratory disease in children with well-controlled symptoms. Moreover, some studies on its preventive effect in patients with subclinical sensitization have begun.28
The aim of this publication is to show the advances of AIT in the treatment of allergic respiratory diseases in children and adolescents. Therefore translational and clinical articles published since 2000 were reviewed, establishing evidence categories to support the strength of the recommendations.
The first part of the article covers the prerequisite issues to understand how AIT is effective, such as the correct etiologic and clinical diagnosis of allergic respiratory diseases, the role of traditional methods of diagnosis in vivo and in vitro, the significance of the molecular diagnosis, the specification of the features of each allergen source together with other factors relevant to the diagnosis, and the appropriate selection of patients and allergens for AIT. In addition, the indications and contraindications of AIT, together with the attitude in patients with comorbidities, are considered in the light of current evidence.
Following this, the article outlines the advancements in understanding the mechanisms by which AIT achieve immune tolerance to allergens. Administration routes, treatment regimens, dose and duration, factors associated with adherence and the determinants of effectiveness, such as the efficacy in clinical and immunological response are also reviewed. Safety is evaluated according to the evidence, highlighting the factors associated with adverse effects, premedication indications and adjustments to the treatment regimens.
Finally, the article reviews advances in the research of AIT, such as improvements in efficacy while preserving safety, new pathways, vectors, immunopotentiating adjuvants, new forms of modified natural allergens and recombinant allergens and epitopes that along with the molecular diagnostics increase the specificity of the treatment for each patient for a more personalized AIT.
Materials and methodsSearch criteriaTo be able to carry out the literature search of the most appropriate and representative articles for each of the topics, the following criteria were established: original articles, reviews, meta-analyses, clinical practice guidelines, expert consensus and clinical trials published since 2000 in both English and Spanish, which included patients up to 18 years of age. In addition, the experts selected the following keywords from each one of the areas, and the search was performed as follows:
- •
Diagnosis:
- ∘
(Component-resolved diagnosis AND Immunotherapy) OR (Molecular diagnosis AND Allergy) OR Sensitization aeroallergens OR (Molecular diagnostic AND Algorithm) OR (In vitro testing AND aeroallergens) OR (Skin-prick testing AND aeroallergens) OR Specific IgE test OR Multiallergen IgE assay OR (Cross reaction AND aeroallergens) OR Geographic sensitization.
- ∘
- •
Patient selection:
- ∘
Allergen immunotherapy AND (Absolute contraindication OR Adverse effects OR (Age AND Indications) OR Allergen-specific immunotherapy OR (Asthma AND (Severe OR Moderate OR Intermittent OR Persistent)) OR Atopic dermatitis OR Autoimmunity OR Clinical aspect for SCIT OR Clinical aspects for SLIT OR Clinical indications Or Contraindication OR Criteria of detection OR Food allergy OR Immunodeficiency OR Immunological disease OR Indication OR Lower age limit OR No indication OR Practice patterns OR (Prevention AND Allergic diseases) OR (Prevention AND asthma) OR (Prevention AND sensitization AND allergens) OR Principles OR Protocol OR Recommendation patterns OR Relative contraindication OR (Rhinitis AND (Severe OR Moderate OR Intermittent OR Persistent)) OR Risk/benefit ratio OR Side effects).
- ∘
Allergen immunotherapy AND Adverse systemic reaction AND (Adverse effects OR Factors associated OR Predictors of side effects OR Predictors of systemic reactions OR Risk factors OR Side effects OR Toxicity).
- ∘
- •
Treatment:
- ∘
Mechanism of action AND (Allergen OR Allergy vaccine OR Dendritic cell OR i.e., antibody OR IgG4 OR IL-10 OR Immunomodulation OR Immunotherapy OR Langerhans cells OR Regulatory T lymphocyte OR SCIT OR SLIT OR Subcutaneous OR Sublingual OR TGF-beta OR Th1 OR Th2 OR Tolerance).
- ∘
Immunotherapy AND (Duration of efficacy OR Long-term efficacy OR Short-term efficacy).
- ∘
Immunotherapy AND efficacy AND (Allergens OR Asthma OR Asthma prevention OR Conjunctivitis OR Efficacy variables OR Immunoglobulin OR Improvement in quality of life OR Medication score OR Prevention of new sensitizations OR Preventive effects OR Pulmonary function OR Quality of life OR Rhinitis OR SCIT OR Skin test OR SLIT OR Symptoms score OR Time to reach the efficacy OR Types of immunotherapy).
- ∘
Types of immunotherapy AND (Allergenic mixtures OR Allergenic sources OR Aqueous allergenic extract OR (Immunotherapy AND omalizumab) OR Immunotherapy composition OR (Modified extract AND (Physical modification OR Depot OR Chemical modification OR Allergoid OR Polymerized OR Mixed modification OR Combined modification)) OR Native allergenic extract OR Oral OR Subcutaneous OR Sublingual OR Types of allergenic extract).
- ∘
Allergen immunotherapy AND (Adjuvant adverse effects OR Adjuvant vaccines OR Advances OR Adverse effects OR Booster OR Build up phase OR Cluster OR Discontinuation OR Dose OR Dose adjustment OR Dose ranges OR Duration OR End of treatment OR Epicutaneous route OR Future Or Immunomodulation OR Immunopotentiator OR Intralymphatic route OR Long-term efficacy OR Maintenance phase OR Missing doses OR Modified Schedule OR Optimal dose OR Peptides OR Practice patterns OR Recommendation patterns OR Regimen OR Relapse OR Rush OR Schedule OR Side effects OR Toll receptors OR Toxicity OR Ultrarush OR Up-dosing OR Vector system).
- ∘
Allergen AND Immunotherapy AND (Subcutaneous OR Sublingual) AND (Adverse effects OR (Adverse systemic reaction AND (Grading system OR Severity)) OR Anaphylaxis OR Angioedema OR Atopic dermatitis OR Biphasic reaction OR Delayed local reaction OR Delayed systemic reaction OR Epinephrine-treatment reactions) OR Fatal reaction OR Immediate local reaction OR Immediate systemic reaction OR Incidence of adverse reaction OR Local reaction OR Non-fatal systemic reaction OR Prevalence of adverse reaction OR Safety OR Severe reaction OR (Tolerability OR tolerance OR systemic tolerability) OR Tryptase OR Urticaria.
- ∘
Allergen immunotherapy AND Adverse systemic reaction AND (Factors associated OR Predictors of side effects OR Predictors of systemic reactions OR Risk factors).
- ∘
Follow-up AND (Adherence OR Allergen OR Compliance OR Efficacy OR Factors OR Gender OR Immunotherapy OR Socio-demographic OR Variables).
- ∘
From the keywords selected, 520 PubMed articles were initially located, of which 480 were chosen based on their significance. These were then studied in depth to extract the available evidence regarding this treatment. Finally, during the analysis carried out, the less relevant studies were discarded and others from more recent publications were added compiling a medical literature of 333 articles.
Oxford classificationThe evidence grading scales, which were generated in an attempt to answer different clinical questions,29 have been used for decades and have been widely criticized over this period of time.30–33 The first hierarchies were created as a means of assisting clinicians and researchers in evaluating the quality of the evidence from the therapeutic effects of the drugs, while the more recent classifications were designed to orient systematic reviewers29 and those who develop the clinical practice guides.34
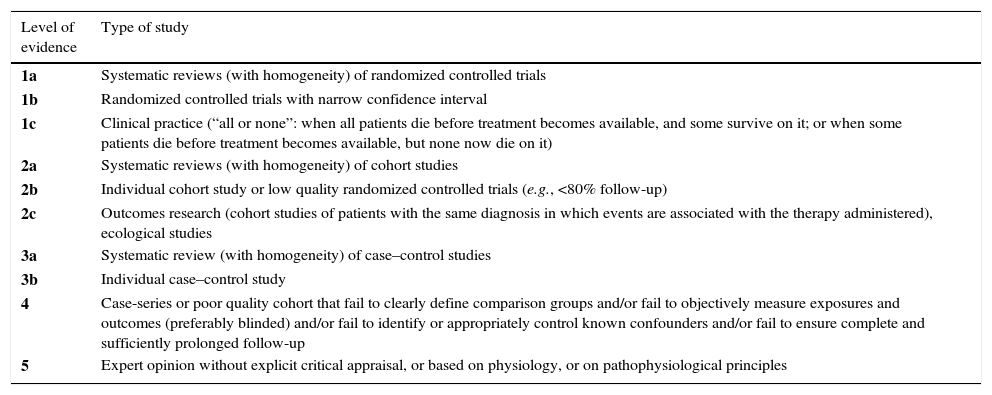
The levels of evidence of the Oxford Centre for Evidence Based Medicine (OCEBM) were first published in September 2000 and reviewed later in May 2011, whose results are shown in Tables 1 and 2. These levels of evidence were designed to provide not only a traditional critical evaluation, but also a heuristic approach so that clinicians and researchers could answer clinical questions quickly, systematically and without having to resort to other sources.
OCEBM levels of evidence.
| Level of evidence | Type of study |
|---|---|
| 1a | Systematic reviews (with homogeneity) of randomized controlled trials |
| 1b | Randomized controlled trials with narrow confidence interval |
| 1c | Clinical practice (“all or none”: when all patients die before treatment becomes available, and some survive on it; or when some patients die before treatment becomes available, but none now die on it) |
| 2a | Systematic reviews (with homogeneity) of cohort studies |
| 2b | Individual cohort study or low quality randomized controlled trials (e.g., <80% follow-up) |
| 2c | Outcomes research (cohort studies of patients with the same diagnosis in which events are associated with the therapy administered), ecological studies |
| 3a | Systematic review (with homogeneity) of case–control studies |
| 3b | Individual case–control study |
| 4 | Case-series or poor quality cohort that fail to clearly define comparison groups and/or fail to objectively measure exposures and outcomes (preferably blinded) and/or fail to identify or appropriately control known confounders and/or fail to ensure complete and sufficiently prolonged follow-up |
| 5 | Expert opinion without explicit critical appraisal, or based on physiology, or on pathophysiological principles |
A minus sign (−) can be added to show that the level fails to provide conclusive evidence in the following cases:
- •
A randomized clinical trial with wide confidence interval and no statistical significance.
- •
A systematic review with statistically significant heterogeneity.
OCEMB grades of recommendation.
| Grades of recommendations | Definition | Level of evidence |
|---|---|---|
| A | Highly recommendable | Level 1 studies |
| B | Favourable | Level 2–3 studies or extrapolation from level 1 studies |
| C | Favourable but not conclusive | Level 4 studies or extrapolation from level 2–3 studies |
| D | Neither recommended nor disapproved | Level 5 studies or inconclusive studies at any level |
Extrapolation is applied when data from our clinical scenarios has important differences concerning the original study situation.
One distinctive characteristic of this classification system is that its levels cover the full range of clinical questions in the order (from top to bottom) needed by the clinician. While most of the grading scales consider the level of evidence with regards to the effects and therapeutic dangers, the OCEBM system allows for the evidence to be evaluated based on the prevalence, accuracy of the diagnostic tests, prognosis, therapeutic effects, adverse effects and the use of early screening.35 For this reason, the group of experts of this work chose to use these levels of evidence and grades of recommendations when establishing those for the sublingual immunotherapy treatment.
DiagnosisEtiologic diagnosisThe accuracy of the allergy diagnosis allows for the selection of good candidates for AIT and for the identification of relevant sensitizing allergen(s) responsible for clinical symptoms, which could improve outcomes and cost efficacy of AIT36,37(level of evidence 1a, grade of recommendation A).
The diagnosis of allergic disease is performed by skin prick tests (SPT) (in vivo test) or through the measurement of specific immunoglobulin E (IgE) in a blood sample (in vitro test). Allergy test results (skin or blood) should always be interpreted in the context of the patient's clinical symptoms, age, relevant allergen exposure and the performance characteristics (sensitivity, specificity, reproducibility) of the allergy tests. Allergic symptoms due to an allergen exposure can be difficult to interpret because of overlapping seasons and multiple allergens in a certain environment. Therefore, allergy diagnosis tests help clinicians to make an accurate diagnosis to identify the causative allergen(s)37,38(level of evidence 1a, grade of recommendation A).
1.1.1Key pointsBefore reviewing tests for allergy diagnosis in allergic respiratory diseases, it is relevant to consider several issues.
Sensitization versus clinical allergyAll allergy diagnostic tests (in vivo and in vitro tests, even molecular diagnosis) should be evaluated with regard to the patient's clinical history. The results of in vivo and/or in vitro tests have to explain the symptoms related to allergen exposure establishing the clinical relevance of sensitization and the allergen(s) to consider36,37(level of evidence 1a, grade of recommendation B).
Several factors have been involved in the presence of symptoms in a sensitized patient such as allergen, levels of total IgE, specific IgE and/or IgG, the epitope specificity of IgE and mono- or poly-sensitization.39 The higher the specific IgE level, the higher the probability of clinical reactions; however, lipid transfer proteins (LTP) could induce severe reactions at low specific IgE concentrations40 and cross-reactive carbohydrate determinants (CCD) could not result in any clinical reactions despite high specific IgE concentrations.41 Sensitization to one or more allergens does not indicate a clinically relevant allergy of each one.42
Single versus multi-allergensAIT formulation with single or few allergens is safe and effective in poly-sensitized patients, whereas multiallergen AIT requires more evidence42(level of evidence 2a, grade of recommendation B). A prescription of AIT is recommended for each allergen when patient is sensitized to several allergens sources and not to administer AIT when sensitization to cross-reactive components. The efficacy of AIT likely depends on the identification of responsible specific allergen(s) of an allergen source and on the concentration of this specific allergen(s) in the extract used43(level of evidence 1a, grade of recommendation A).
Definition of allergen source, allergen molecules, cross-sensitization and co-sensitizationAllergen source is a tissue, particle, food or organism able to elicit allergy (e.g., cat, mite, milk, Aspergillus fumigatus). Allergen extract is obtained from an allergen source (e.g., pollen grains) and consists of a mixture (in variable proportions) of allergenic and non-allergenic proteins, polysaccharides and lipids. The composition and relative concentration of all allergens in natural extracts for AIT and for diagnosis are unknown; relevant allergens can be present in small amounts and have variable biological potency.44
Allergen component or molecule is a protein or glycoprotein able to bind IgE.45 Epitopes are unique regions on the surface of an antigen/protein that are able of binding IgE and eliciting immune response. There are commonly several different epitopes on each allergen component. Many different molecules share common epitopes and the same IgE antibody can bind and induce an immune response to allergenic molecules with similar structures from various allergen sources. In contrast, some molecules are unique markers for specific allergen sources. Cross-sensitization or cross-reactivity occurs when the same IgE binds to several allergens with common structural features (usually more than 50–70% amino acid sequence identity). Some cross-reacting molecules can cause clinically relevant symptoms, while others usually do not38(level of evidence 1a, grade of recommendation A).
Allergens can be isolated from natural allergen sources (native, purified allergen) or can be produced by using recombinant DNA technology (recombinant allergen). The abundance of a molecule present in the allergen source is relevant when clinicians consider AIT. A child with respiratory allergy may be sensitized and/or clinically allergic to one or more allergens. Poly-sensitization has been defined as sensitization to two or more allergens (e.g., mite, olive and grass pollen); and when clinical symptoms related to these allergens are present, the term of poly-allergy can be used.42
1.1.2TestsThere is currently no gold standard test for diagnosing aeroallergen allergy.46 Detection of IgE of a specific allergen in the skin and blood simply provides confirmation of sensitization, not allergic disease46(level of evidence 1a, grade of recommendation A).
It is important to know the performance and limitations of each testing method in predicting clinical disease in response to the allergen(s). Most studies comparing in vitro tests and SPT indicate that SPT is more sensitive (lower false negative rate) and specific IgE tests to be more specific (lower false positive rate).47 Both skin and in vitro IgE tests may commonly be associated with false-positive results, whereas false-negative results may rarely occur. SPT and specific IgE testing should be considered as complementary to each other and should not be interpreted interchangeably. It may not always be necessary to apply both SPT and specific IgE testing in clinical practice with individualized choice of the diagnostic testing method.
In vivo testsThe SPT is based on crude extracts composed of allergenic and non-allergenic molecules obtained from an allergenic source. The consensus of the Global Allergy and Asthma European Network (GA2LEN) and AR and its Impact on Asthma (ARIA) established recommendations on the use of SPT in AR-conjunctivitis and asthma in clinical practice.38 In brief, they recommended standardized extracts when available, a positive and a negative control solution, tests on normal skin (without eczema), to evaluate dermographism, to ask for medications taken by the patient and time of last dose, and to measure the longest wheal diameter after 15min.
SPT is highly specific (70–95%) and sensitive (80–97%) for the diagnosis of sensitization to inhalant allergens with a high degree of correlation with symptoms. The positive predictive value for Dermatophagoides pteronyssinus ranges from 77% to 100% for younger subjects. Wheal diameters≥3mm are considered positive. Although larger reaction was considered not to be associated with more severe disease, a study found a relationship of more frequent symptoms in case of larger wheals.48 Depending on the allergen, from 40% (Blatella sp.) to 87–89% (grass, mites) of the positive SPT reactions (wheal size≥3mm) were associated with patient-reported clinical symptoms when exposed to the respective allergen. Children with positive SPT reactions had a smaller risk of sensitizations being clinically relevant compared with adults.49
Characterization and standardization of allergen concentrations in allergen extracts are crucial in the interpretation of results. When extracts are poorly extracted, degraded or inactivated during the preparation, the major allergen may be present in low amounts or even absent. Variations in the quality and/or potency (decrease over time) of commercially available extracts exist. SPT results obtained with the same allergen source with extracts from different manufacturers vary.50 In addition, not all patients are allergic to all allergen extract content. A European project encourages the standardization of allergen extracts based on their content of major allergen(s),51 such as the use in research of recombinant allergen extracts with a limited number of allergens.52
SPT can be used from infancy to all paediatric age.53 Repeated testing may be needed to detect new sensitizations in children and if symptoms change. A common standardized allergen battery (18 allergens) for Europe has been recommended based on the GA2LEN study.54 However, the panel should always include local allergens.
SPT and specific IgE do not have the same biological and clinical relevance, and are not interchangeable. Concordance between in vitro specific IgE assays and SPT results is 85–95%, depending on the allergen being tested55 and the method used to detect specific IgE.36 In a study of 8000 subjects, SPT versus specific IgE with the CAP-FEIA® technology (Thermo Fisher Scientific, Uppsala, Sweden) showed the best positive predictive value to determine clinical allergy for respiratory allergic diseases.
Some allergens exhibit poor biological activity and skin testing may not be useful to identify such allergens. Improper technique (weak puncture) and limited local production of allergen specific IgE has been associated with false negative SPT. Positive mite SPT and negative specific IgE to mite allergen could indicate that other additional allergen components are needed (e.g., allergen mites of groups 6, 9, 11 and 12). Positive specific IgE to components with negative SPT could suggest an insufficient amount of allergenic components in the crude extract. Skin test reactivity may decrease with AIT to inhalant allergens, but SPT cannot be used to assess the efficacy of AIT in clinical practice53(level of evidence 1a, grade of recommendation B).
Variability in SPT results may be due to several factors: (1) age (reactivity peaks in the late teens to early 20s and then decreases over time); (2) histamine sensitivity (inherent inborn sensitivity may increase or decrease skin test reactivity); (3) chronobiology (circadian and circannual variability); (4) testing device; (5) extract quality (weaker extracts: false-negative results); (6) location of body (back, arm) and test (e.g., adjacent testing to a strong positive extract may produce a false-positive result).
Advantages of SPT:
- 1.
High sensitivity in some extracts compared to allergen molecules.
- 2.
Immediate reading results of many different allergens simultaneously.
- 3.
Minimally invasive.
- 4.
Inexpensive.
Disadvantages of SPT:
- 1.
Some allergens can be poorly represented in extracts because of the biological variability of the allergen source or even absent (e.g., Can f 5, for dog allergy).
- 2.
Presence of cross-reactive allergens in the diagnostic extracts such as profilins, polcalcins, LTP, PR10 and tropomyosins, which may produce multiple positive results [e.g., sensitization to grass pollen could also test positive for birch because the birch extract used in SPT contains profilin (Bet v 2) homologous to profilin in grass (Phl p 12)].
- 3.
Manual technique (variability) with good reproducibility when SPT is performed correctly by trained health professionals.
- 4.
Not appropriate for monitoring sensitization.
- 5.
Crude extracts.
In summary, SPT is an easy quick test, which generally have better overall predictability than in vitro tests and is the preferred initial diagnostic approach53(level of evidence 1a, grade of recommendation A). It should be appropriately interpreted based on clinical symptoms, and other tests may be necessary in order to assess a specific allergen.
In vitro testsAn allergy blood test detects and measures circulating IgE, which is directed at a specific allergen molecule or extract. The specific IgE threshold that indicates the presence or absence of clinical symptoms is not known. Low levels of specific IgE are less often associated with symptoms than higher levels, but they do not exclude allergic symptoms, especially in very young children.56 In subjects with very high total IgE, low levels of specific IgE of doubtful clinical relevance are often detected. Higher total IgE levels lower the specificity and predictive positive value (when compared with SPT), so total IgE levels should obtained at the same time as specific IgE testing47(level of evidence 1a, grade of recommendation A).
Aeroallergen sensitization detected by specific IgE testing with corresponding negative SPT is more likely in individuals with high total specific IgE. A study of 38 children (age 1.5–3 years) found that children with total specific IgE>300kU/L were twice as likely to have SPT-negative/specific IgE-positive “mismatches” than children with total specific IgE<300kU/L.57 Conversely, children with high specific IgE were much less likely to have SPT-positive/specific IgE-negative results compared to those with low specific IgE.57 Another study evaluated the agreement between specific IgE and SPT and the possible association between total IgE concentration and clinical symptoms in an unselected cohort of 353 children aged two year old. The lack of agreement between both methods for positive tests in some allergens suggested that both tests should be used in a complementarily way in young children58(level of evidence 1b, grade of recommendation B).
Specific IgE testing is preferred to SPT for uncooperative patients and when the patient has severe skin disease (extensive eczema, dermographism, urticaria), is receiving medications that may suppress skin tests and cannot be removed from them.
There are two main in vitro tests: specific IgE to allergen extracts and molecular diagnosis.
- a)
Specific IgE to allergen extracts: This method is based on crude extracts composed of allergenic and non-allergenic molecules obtained from an allergenic source. It cannot identify cross-reacting molecules. ImmunoCAP® (Thermo Fisher Scientific, Uppsala, Sweden) is the assay most extensively studied.
Measurements are reported in arbitrary mass units (kilo international units of allergen specific antibody per unit volume of sample [kUa/L]). In ImmunoCAP system, 1 international unit is equal to 2.42ng of specific IgE. The performance of each in vitro assay of specific IgE is different as manufacturers often modify allergens or reagents. In vitro methods may not yield comparable results, even if they are reported in the same units.
A study comparing two in vitro tests (chemiluminescent assay [CLA] and capsulated hydrophilic carrier polymer systems) with SPT showed that the specificity of both tests was low but with similar sensitivity (high).59 This indicated that positive in vitro test results should be evaluated carefully taken into account symptoms, exposure and SPT to determine the clinical relevance of the allergen sensitization. Specificity was allergen dependent, and in vitro tests reported a greater number of positive tests to mites than SPT.59
Specific IgE had a high negative predictive value of negative test results. In patients with symptoms, SPT-positive and negative in vitro test may be due to difference in extract allergen composition.59 Some allergens have SPT-positive results and specific IgE-negative results against extract of cypress, timothy, grass, ragweed, Russian thistle and mugwort. In contrast, two allergens of dog extract have showed SPT-negative and specific IgE-positive results against the extract.47
The number of sensitizations that can be missed if either of the testing methods are being used alone has been evaluated in a large multicentre study.60 Among dust mite-sensitized participants only 58% were both SPT and specific IgE positive for dust mite; 21% of dust mite sensitizations would have been missed if specific IgE or SPT tests are evaluated separately (similar results for timothy grass). An extreme discrepancy between SPT and specific IgE was found for Cladosporium sp., with 8% of specific IgE-positive and SPT-negative; 58% would have been missed with SPT alone, and 34% of sensitizations would have been missed with specific IgE testing alone.
There is a considerable overlap of total IgE values in healthy and allergic subjects being total IgE a poor screening test for sensitization61,62(level of evidence 1a, grade of recommendation A).
Advantages of specific IgE testing:
- 1.
Automatic method.
- 2.
Quantitative assay.
- 3.
High sensitivity.
- 4.
Lower variability.
- 5.
It uses natural or recombinants proteins or crude extracts.
- 6.
To assess monitoring sensitization and timing of natural exposure to allergen (some cases).
- 7.
To use in patients in whom SPT cannot be performed.
Disadvantages of specific IgE testing:
- 1.
It needs approximately 40μl of serum per each allergen tested.
- 2.
It only performs one allergen per assay.
- 3.
It detects low-affinity IgE that may have no clinical relevance.
- 1.
- b)
Molecular diagnosis: This is an in vitro method that measures specific IgE that binds to single allergenic protein components (purified from natural sources or obtained by recombinant techniques) or even peptide fragments of allergenic proteins rather than whole allergen extracts.38,63 Specific allergens are markers for their respective allergen sources and others are markers of cross-reactivity. Molecular diagnosis can establish the probability of patient symptoms due to exposure to different allergen sources by the pattern of sensitization to different allergens.
In clinical practice, with patients sensitized to different pollen species, molecular diagnosis was able to improve the resolution of conventional diagnostic tests (SPT and/or specific IgE based on extracts) in a substantial number of cases, either by detecting new relevant sensitizations or by ruling out clinically irrelevant sensitizations caused by non-symptomatic cross-reactive allergens64–67(level of evidence 1b, grade of recommendation A). Molecular diagnosis has somewhat lower sensitivity of individual allergen diagnostics with respect to many allergens, and not everything that is possible to diagnose at present is sensible for use in routine diagnosis. However, molecular diagnosis avoids the recognition of low-affinity IgE in lower allergen amounts.
It is not clear if recombinant forms are equivalent with their natural forms. Purified natural allergens (not recombinant molecules) may contain glycoproteins (CCD) that could result positive due to cross-reactivity. When glycosylated allergen molecules are used (as natural purified glycoproteins), specific IgE to CCD could drive a positive result in in vitro tests based on extracts and in tests based on allergenic molecules (molecular diagnosis). The recombinant forms of the proteins are not glycosylated but may theoretically result in improper folding of the allergen protein, driving a false negative result. To exclude the presence of IgE against only the carbohydrate moiety in the absence of specific IgE against the protein fragment is recommended to use markers of CCD, such as bromelain (nAna c 2) and MUXF3. These markers are able to detect N-glycans in most pollen sources. For nArt v 1, which contain O-glycans, CCD nAna c 2 and MUXF3 are not useful. Therefore, positive result to natural glycoproteins allergen molecules with negative result to CCD markers would suggest sensitization to the protein (e.g., nCup a 1-positive and MUXF3-negative indicates sensitization to cypress). Positive result to CCD markers needs to demonstrate the biological activity of the specific IgE to protein.
Singleplex and multiplex measurement platforms are available for identifying IgE against allergenic molecules in the molecular diagnosis. Singleplex consists of one assay per sample. The clinicians must choose those allergenic molecules necessary for an accurate diagnosis defined by patient's clinical history. Taking into account that including a higher number of molecules increases the economic cost, clinicians must have to consider if it is better to use a multiplex test instead (e.g., for Phleum pratense, Phl p 1 and Phl p 5 is enough for most patients; adding Phl p 2 and Phl p 4 could improve the accuracy of the diagnosis of grass sensitization). If more than 10 to 12 allergens are required for an accurate diagnosis using singleplex tests, then a multiplex test may be preferable for economic reasons68(level of evidence 1b, grade of recommendation A). The singleplex platforms commercially available are ImmunoCAP®, ImmuLite® and HyTech® (GardenGrove, California). These platforms use panels of single allergens. The detection limit of these systems is usually 0.35kU/L specific IgE. The most used is ImmunoCAP, which measures quantitatively specific IgE levels (kU/L).
The usefulness of molecular diagnosis in childhood allergies has been evaluated in a study of 162 children aged 4–16 years diagnosed with allergic pollen rhinitis or asthma/rhinitis (clinical history and positive SPT).69 They compared specific IgE against pollen allergens versus P. pratense allergen molecules (Phl p 1+Phl p 5 as P. pratense-specific allergens, and Phl p 7+Phl p 12 as cross-reacting allergens). Sensitization to Phl p 1+Phl p 5 was detected in 99.4% and cross-reacting allergens in 46% (Phl p 7+Phl p 12). Multiple sensitizations to pollen were documented in 38% of patients (Plantago lanceolata most common cause). Children with negative results for Phl p 1+Phl p 5 revealed positive values for cross-reacting allergens and Plantago sp. and Chenopodium sp. pollens. Sensitization to major allergens is correlated with sensitization to cross-reacting allergens, with most children sensitized to both specific and cross-reacting molecules (Phl p 1+Phl p 5 and Phl p 7+Phl p 12). Another important finding of this study is that the specific IgE values for the cross-reacting allergens are significantly lower than those of the major allergens, which could be regarded as the cause of sensitization to grasses. This reinforces the fact that to quantify specific IgE is also relevant in molecular diagnosis as quantification specific IgE may objectively establish associations between groups of allergens for diagnostic purposes38(level of evidence 1b, grade of recommendation A). However, higher or more frequent specific IgE has been observed with some molecules not commonly found in the environment. Noteworthy, Scala et al. suggested that these components could be good reagents rather than the first sensitizer molecule of the family.70
ADVIA-Centaur® (Bayer-HealthCare Diagnostics Division, Tarrytown, New York) is a singleplex molecular diagnosis test that detects specific IgE to individual allergen molecules. The ADVIA-Centaur® specific IgE assay is a reverse sandwich immunoassay using direct CLA. It is able to exclude responses from low-affinity IgE. The main advantages of this method are the use of only a small quantity of serum (25μl per allergen versus 40μl for the UniCAP system), rapid turnaround time, complete automation (hands-on time 10min for Centaur versus 1.5h for UniCAP) and no interference from IgG.71
Multiplex platforms consist of multiple assays per sample with a broad array of pre-selected allergens on a chip independently of the clinical history. The Immuno-Solid phase Allergen Chip (ISAC) is the most comprehensive multiplex platform currently available, with more than 100 commercial allergenic molecules from about 50 allergen sources. The allergens are spotted in triplicate and covalently immobilized on the chip. Two negative spots are considered negative result. Results are reported within a range of 0.3–100 ISAC Standardized Units (ISU), a semi-quantitative measure (signal intensity) different from results of ImmunoCAP (kU/L). Although the ISAC results are similar with those obtained from singleplex platforms, they are not interchangeable. Both tests correlate well despite concordance of results vary between allergens tested36,64,72(level of evidence 1b, grade of recommendation A).
The concordance of results between allergens tested is lower for positive results than for negative results (78.65% versus 93.57%, respectively).73 At low specific IgE levels, ImmunoCAP is more sensitive than ISAC. ImmunoCAP technology measures IgE binding under conditions of excess of immobilized allergen whereas ISAC uses low amounts of immobilized allergen allowing for competition with allergen-specific isotypes (e.g., IgG) other than IgE. Specific blocking IgG are induced by AIT and those IgG could interfere the specific IgE levels detected by ISAC; thus, it has been suggested to use multiplex platform as an indirect measurement of AIT efficacy.74 High levels of total IgE do not interfere in ISAC platform. For some allergens, there is a higher degree of assay variability for ISAC than of ImmunoCAP so that ISAC has not been recommended to monitor quantitative IgE levels over time in clinical routine38(level of evidence 1b, grade of recommendation A).
ISAC is especially suited for use in patients with complex sensitization pattern or symptoms (when sensitization to cross-reacting allergens is suspected and when both food and airborne allergens are involved) (level of evidence 1b, grade of recommendation A). A clinical study demonstrated that molecular diagnosis changed the indications and type of AIT prescription in 54% of patients with pollen AR in an area of complex pollen sensitization.65 The change of AIT was based on molecular diagnosis compared to SPT and/or specific IgE with commercial extracts. Sensitization to profilins or polcalcins showed the poorest agreement between SPT and ISAC.65 Molecular diagnosis is not only very useful in poly-sensitized patients, but also when clinicians have not suspected allergen(s) as ISAC contains a broad allergen panel.
Results obtained with ISAC platform are similar to those obtained with the ImmunoCAP platform. Singleplex tests are more quantitative (kU/L) than multiplex test (ISAC) that uses semi-quantitative units (ISU). Although ISAC has lower sensitivity and higher variability at low specific IgE levels (0.3–1ISU) and likely less clinical relevance, it is able to identify with a small quantity of serum a broad panel of allergens suspected and unsuspected. The performance of ISAC platform with higher IgE levels is correct, unlike what happens with ImmunoCAP.
Advantages of molecular diagnosis:
- 1.
It assesses the appropriate and individualized indication for AIT and selects the optimal allergen(s).
- 2.
It provides extensive sensitization profile.
- 3.
It identifies sensitization patterns associated with prognostic outcomes (assessment of severity of reaction associated with certain allergens).
- 4.
It predicts the risk of adverse reactions of AIT.
- 5.
It distinguishes between cross-reactivity and co-sensitization, and understands patient symptoms due to this phenomenon. It allows determining whether a single, a few closely related or several widely different allergen sources need to be considered.
- 6.
In the case of cross-reactive allergens, it gives information on potential sensitization and clinical reactions to several different sources, even unanticipated or potentially high risky allergens.
- 7.
It tests a large number of allergens (natural or recombinant molecules) using a small amount of serum (ISAC platform).
- 8.
Less allergen is needed per assay without interference at high total IgE level (ISAC).
- 9.
It studies sensitization at early stage and progression to a clinical stage allowing knowing the sensitization or allergic march.
Disadvantages of molecular diagnosis:
- 1.
Not all allergenic sources are included. It is needed expansion of additional allergenic molecules.
- 2.
Interpretation of the results can be confused. The clinical utility of many allergenic molecules needs further investigation. Guidelines for appropriate test interpretation are needed each time new molecule is discovered.
- 3.
Detection of some cross-reactive molecules without knowledge of their underlying mechanism causing cross-reactivity and symptom presentation.
- 4.
The ISAC platform has higher degree of variability in low levels of specific IgE (0.3–1 ISU-E); other isotypes (e.g., IgG) can potentially interfere results. This method is a semi-quantitative and manual assay.
- 5.
Lower sensitivity of ISAC platform compared to ImmunoCAP.
- 6.
The results in ISAC platform are variable and therefore it is not recommended for monitoring disease or response to treatments.
- 7.
ImmunoCAP has less allergens molecules available.
Current guidelines of allergy diagnosis recommend a thorough clinical history investigation as a first step, followed by allergen extract testing using in vivo SPT and/or in vitro specific IgE for the identification of the allergen source responsible for a patient's symptoms. Molecular diagnosis is recommended as a third step when previous tests were inconclusive38(level of evidence 1a, grade of recommendation A), although experienced clinicians may also use it as a second step test. An algorithm using a panel of specific markers of allergen sources and a panallergen screening has been proposed to assess patients from southern Europe suitable for AIT based on extracts.44 They recommend in mono-oligo-sensitization patients assessing sensitization to specific major molecules contained in AIT extracts. For poly-sensitized patients, they recommend to determine panallergens such as specific IgE against profilins/polcalcins that could suggest a poor outcome for AIT. If non-glycosylated recombinant molecules are used, sensitization to rPhl p 5/rPhl p 1 (grass), rPar j 2 (Parietaria sp.) or rOle e 1 (olive) may suggest a good outcome for AIT. If the results are positive to purified natural CCD-contained forms such as nCyn d 1 (Bermuda grass), nCup a 1 (cypress), nOle e 1 (olive), nSal k 1 (salwort) and nArt v 1 (mugwort), they suggest to rule out sensitization only to CCD or to elucidate concurrent sensitization to both glycosylated and protein parts. Recently, a User's Guide for Molecular Allergology has been published by the European Academy of Allergy and Clinical Immunology which includes the interpretation of molecular diagnosis results in order to make a clinical decision about suitable AIT.75
In summary, molecular diagnosis allows determining the indication and the optimal allergen(s) for AIT because is able to distinguish between sensitization to specific unique molecule of an allergen source and sensitization to highly cross-reactive molecules. The usefulness is high in poly-sensitized patients, with unclear symptoms related to exposure or without response to treatment. Mono-sensitized patients with a clear clinical history related to exposure could be diagnosed with in vivo, in vitro or both diagnostic tests based on allergen extracts.
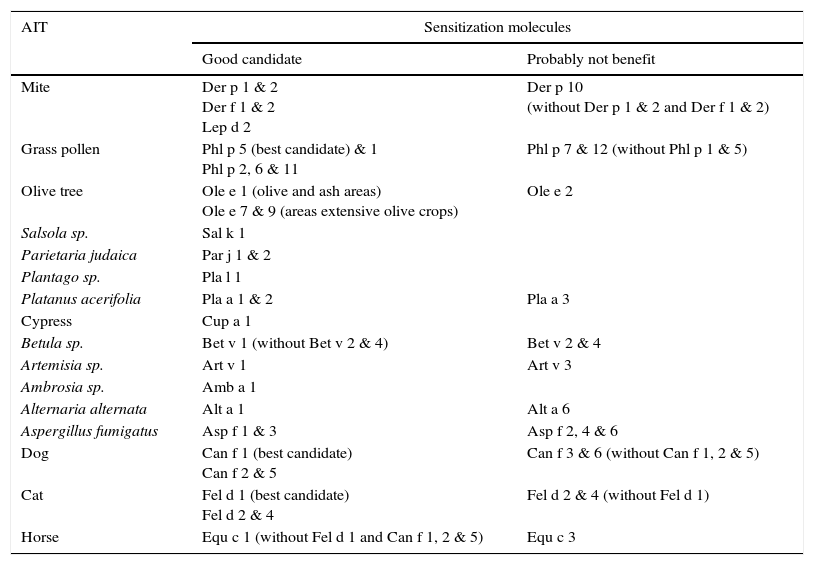
1.1.3Relevant inhalant allergen molecules for AITThe allergen molecules useful for identification of those patients best suited for AIT are depicted in Table 3. Specific IgE to the same major allergen used in extract standardization (diagnostic and therapeutic) would increase the potential response to AIT76(level of evidence 1a, grade of recommendation A). Although profilins and polcalcins can trigger symptoms, they are not presented in high quantity in extracts AIT. Sensitization to profilins (rPhl p 12 and rBet v 2) and polcalcins (rPhl p 7 and rBet v 4) are markers of cross-reactivity and representative of the entire group of homologous proteins except of Parietaria sp. and cypress profilins. Profilin and polcalcin grass are the most frequent cause of sensitization to panallergens with high cross-reactivity. Sensitization to Phl p 12 and Phl p 7 could indicate a poor outcome in pollen AIT. In areas with high prevalence of birch pollen rBet v 4 and rBet v 2 can be used as markers of cross-reactivity (not in Mediterranean regions).
Relevant inhalant allergen molecules for AIT.
| AIT | Sensitization molecules | |
|---|---|---|
| Good candidate | Probably not benefit | |
| Mite | Der p 1 & 2 Der f 1 & 2 Lep d 2 | Der p 10 (without Der p 1 & 2 and Der f 1 & 2) |
| Grass pollen | Phl p 5 (best candidate) & 1 Phl p 2, 6 & 11 | Phl p 7 & 12 (without Phl p 1 & 5) |
| Olive tree | Ole e 1 (olive and ash areas) Ole e 7 & 9 (areas extensive olive crops) | Ole e 2 |
| Salsola sp. | Sal k 1 | |
| Parietaria judaica | Par j 1 & 2 | |
| Plantago sp. | Pla l 1 | |
| Platanus acerifolia | Pla a 1 & 2 | Pla a 3 |
| Cypress | Cup a 1 | |
| Betula sp. | Bet v 1 (without Bet v 2 & 4) | Bet v 2 & 4 |
| Artemisia sp. | Art v 1 | Art v 3 |
| Ambrosia sp. | Amb a 1 | |
| Alternaria alternata | Alt a 1 | Alt a 6 |
| Aspergillus fumigatus | Asp f 1 & 3 | Asp f 2, 4 & 6 |
| Dog | Can f 1 (best candidate) Can f 2 & 5 | Can f 3 & 6 (without Can f 1, 2 & 5) |
| Cat | Fel d 1 (best candidate) Fel d 2 & 4 | Fel d 2 & 4 (without Fel d 1) |
| Horse | Equ c 1 (without Fel d 1 and Can f 1, 2 & 5) | Equ c 3 |
Several factors of the allergic diagnosis of respiratory diseases affect the treatment of allergy and specifically in the decision of AIT.
Allergen and type of allergic sensitizationChildren initially mono-sensitized to mites become poly-sensitized more frequently than children mono-sensitized to pollens (45.4% versus 32.1%, respectively).77 Sensitization to P. pratense at the age of three years predicted presence of rhinitis by the age of 12 years old.39 Simultaneous sensitization to both profilins and polcalcins has been associated with longer duration of allergic disease, progression to sensitization to other molecules from the same source and co-sensitization to several/high allergen molecules from different sources. Although profilins sensitization is usually associated with mild or no clinical symptoms, some patients may develop more severe reactions.78 Sensitization to profilins has been also associated with more severe respiratory symptoms in grass-allergic patients, especially in areas with high levels of grass pollen exposure.79 Poly-sensitization to several different allergens from a single allergen source may increase symptom severity.80,81
GeographicalAeroallergen sensitization profiles and disease expression differ according to local exposures patterns characteristic of the geographical region and genetic differences. Results obtained with allergic tests have to be related to local population studied. Grass is the first sensitization pollen followed by Olea sp. pollen (29.8%) in Spain. The highest prevalence to Par j 1 (Parietaria sp.) has been detected in certain areas of the Atlantic regions and in Tenerife (Canary Islands) and thus it cannot be considered to be only a Mediterranean allergen. In some areas, Pla l 1 is the second allergen after grass. Bet v 1 is relevant only in Galicia.82 Around 80% of the allergic population is sensitized only to grass in the northwestern Atlantic coast of Spain. In contrast, most patients in dry semi-desert areas and on the Mediterranean coast (lower pollen counts) are poly-sensitized showing high specific IgE levels to both major allergens and panallergens.82 In Spain, the overlapping pollinization periods make clinical history unable to identify the relevant allergen(s) for AIT. The GA2LEN skin test study showed that many allergens previously regarded as untypical for some regions in Europe have been underestimated, such as Olea sp. sensitization in Nordic countries.
Severity and type of diseaseAeroallergen sensitization is strongly associated with rhinitis, asthma and conjunctivitis.57 AIT shows more efficacy in moderate-to-severe disease than in mild disease. There is a relationship between sensitization and disease severity with more severe symptoms in poly-sensitized patients than in mono-sensitized.62 Therefore, poly-sensitized patient could be a good candidate for AIT. Some allergen molecules have been associated with severe asthma in children such as Fel d 1 (cat) and Can f 5 (dog)83. Respiratory allergy was associated with both D. pteronyssinus and Dermatophagoides farinae exposure, while sensitization only to D. farinae was associated with atopic dermatitis.84 Sensitivity to Alternaria sp. is a risk factor for development, persistence and exacerbation of asthma in children.
AgePoly-sensitization develops over time and is a risk for respiratory allergy (e.g., 43.6% of 165 mono-sensitized children with asthma became poly-sensitized).77 Children have higher risk of respiratory diseases with lower number of sensitizations than adults; 5 or 6 sensitizations increase the risk of rhinitis (adjusted odds ratio [AOR] 12.73) and 7 or more sensitizations increase the risk of asthma (AOR 6.12) in children85(level of evidence 1b, grade of recommendation B). However, there is not always a relationship between an increase in number of sensitizations and respiratory allergy; some infants with wheezing and sensitization will develop new sensitizations 5 years later without wheezing at this age. Using both methods in vivo (SPT) and in vitro (specific IgE) have been recommended to investigate sensitization in young children as lower concordance is reported at young ages58(level of evidence 2b, grade of recommendation B).
Paediatric population that shared the same geographical area of adults could have different pattern of sensitization. In a population of 66 children from the centre of Spain (mean age 10.32±4.07 years) with rhinoconjuntivitis and/or asthma and positive SPT and/or specific IgE to olive and grass pollen, sensitization to both olive and grasses (co-sensitization) was common. Sensitization to Ole e 1, Phl p 1 and Phl p 5 were present in 94.5%, 94.5% and 58.2% of the patients, respectively. A minority of patients recognized cross-reactivity to allergens.86
Total IgE levels increase in the first 6 months of life and continue to increase in the next two years of life. Specific aeroallergen sensitizations can already be detected within the first year of life in a few children. Up to 18.7% of the children of two year old had one or more positive reactions to SPT and/or specific IgE in a panel of 12 allergens.58 The prevalence of specific aeroallergen sensitizations (i.e., dust mite) seems to steadily rise during the first 7 years of life.87
Efficacy and adverse reactions of AITMost allergen extracts used in AIT contain standardized major allergens with minimal or variable amounts of minor allergens.38 Only one major allergen at high doses is usually ensured in the extract. Patients with sensitization to minor allergens alone will not receive sufficient amounts of allergen to improve their symptoms. Thus, better AIT outcomes have been found in those patients sensitized to the specific unique allergens of birch or grass pollen compared to patients sensitized to only minor, cross-reactive allergens88(level of evidence 1a, grade of recommendation A).
Some allergen sensitization patterns may also predict the risk of AIT adverse reactions. Specific allergens markers of more severe symptoms in pollen allergy and increased risk of systemic reactions during AIT are Ole e 9 and LTP Ole e 779(level of evidence 1b, grade of recommendation B).
1.1.5Etiologic diagnosis summaryIn some patients, a detailed clinical history and tests based on extracts (SPT and/or in vitro specific IgE) are sufficient to identify the relevant allergen(s). However, when patients show poly-sensitization by diagnostic tests based on allergen extracts (SPT and/or in vitro specific IgE) and their clinical history is not enough to clarify the nature of the sensitization, molecular diagnosis is needed to indicate AIT and to determine the relevant allergen(s). ISAC allows the identification of a broad panel of allergen molecules than ImmunoCAP-based on allergen molecules. However, ImmunoCAP shows higher sensitivity at low levels of IgE, specific IgE quantification and less variability.
Patient selection1.1.6IndicationsAIT may only be used in diseases where an IgE mediated allergy mechanism is central to its pathogenesis76(level of evidence 1a, grade of recommendation A). Therefore, in addition to demonstrating allergic sensitization by skin tests or by determination of specific serum IgE, the existence of a correlation between sensitization with the clinical symptoms presented must be ensured. Identifying the allergen responsible for the patient's symptoms is an essential requirement for prescribing treatment with immunotherapy38,59(level of evidence 1a, grade of recommendation B).
Given the current scientific evidence, the clinical histories for which this treatment is indicated are: rhinitis that is not controlled with the usual pharmacological treatment, allergic asthma and allergy to Hymenoptera venom89(level of evidence 1a, grade of recommendation A). AIT is indicated as an adjunct treatment to allergen avoidance measures and drug treatment. Normally, AIT is indicated in patients aged 5–50 years of age, although today it is increasingly recommended in patients under 5 years of age90,91(level of evidence 2a, grade of recommendation B). AIT has shown to be effective in children and is often well tolerated, although parents must always be relied on to properly follow the immunotherapy regimen.92
The decision to initiate AIT treatment may depend on several factors including, but not limited to, the availability of a suitable extract with properly documented efficacy, patient preferences and the degree of compliance predictable, medication needs, the degree of patient exposure, the response to the allergen avoidance measures and the existence of and response to side effects of different drugs. However, other factors that influence the decision to initiate treatment with AIT exist76,89,93(level of evidence 2a, grade of recommendation C), namely:
- •
Patients with symptoms that suggest conjunctivitis, AR or allergic asthma. Patients with atopic dermatitis and sensitization to inhalant allergens could also be assessed.94,95 Patients with mild initial asthma can benefit far more from AIT than those with moderate or severe asthma, which first require stabilization or control of their asthma and also have a higher risk of developing adverse reactions to the AIT.89
- •
Patients presenting with a specific IgE, confirmed by either SPT (the most common and cost-effective method) or by determination of serum specific IgE.96,97
- •
There should also be a correlation between patient symptoms and a sensitization to the allergen exposed. In the case of a patient presenting with positive SPT or specific IgE, but with symptoms that are not clinically consistent and unrelated to exposure, it would be considered an asymptomatic sensitization and therefore not indicated for AIT.89
- •
Patients with insufficient symptom control despite pharmacological measures and allergen avoidance, who require high-dose therapy and experience side effects from the use of multiple drugs or wish to avoid prolonged drug treatment, may be possible candidates for AIT.76,98
- •
The patient's clinical response in terms of quality of life and responsiveness to other forms of treatment, such as avoidance of the allergen or to the pharmacological measures, should also be factors to consider in the decision to prescribe AIT.76
- •
The severity and duration of symptoms should also be taken into account in assessing the need for AIT. The severity of symptoms can be defined by subjective and objective parameters. Time lost from work, visits to the emergency room or doctor's office (indirect costs) and the response to pharmacotherapy are important objective indicators of the severity of the allergic disease. The symptoms that interfere with sleep, work or school performance are other factors to consider.76
- •
The presence of comorbidities must also be considered in the evaluation of a patient as a possible candidate for AIT.76
- •
Patients with AR who suffer from sleep disturbances due to symptoms or whose symptoms interfere with work or school performance are particularly good candidates for AIT.
- •
Patients who experience adverse side effects from pharmacotherapy, such as nosebleeds with intranasal steroids or excessive drowsiness with antihistamines, and those who find the pharmacotherapy inconvenient or ineffective may also be suitable candidates for immunotherapy.99
- •
Patients with coexisting AR and asthma should be treated with an appropriate combination of allergen avoidance measures and drug treatment, but can also benefit from AIT. However, the asthmatic condition of the patient must be stabilized before administering AIT.76,100
For the AIT to be effective, the allergen responsible for the patient's symptoms should be identified and should be an essential requirement for the treatment with immunotherapy38,59(level of evidence 1a, grade of recommendation B). A correlation between symptoms, allergen exposure and the results of diagnostic tests should exist.38,63
The specific aeroallergen responsible for the symptoms should be identified (through SPT, specific IgE and molecular diagnosis), especially in the case of poly-sensitized patients as it differentiates whether poly-sensitization is the result of a true sensitization to various pollens or the effect of cross-reactivity to panallergens63,64,72,73,101–105(level of evidence 2a, grade of recommendation B).
While SPT and specific IgE to extracts only detect the sensitizing source, the molecular diagnosis clearly identifies the responsible allergen. If a molecular diagnosis is not available, an individualized patient assessment based on the experience of the specialist prescribing the immunotherapy should be performed. Otherwise, the indication should be assessed individually. This also applies in the case of allergen mixtures.
Once the patient is considered eligible to receive AIT, the administration route must be selected: subcutaneously (native or modified extract) or sublingually (drops or tablets). Depending on the type of AIT, a set of indications may also be established.106
- •
Subcutaneous immunotherapy treatment (SCIT) is indicated for the following cases:
- ∘
Patients with prolonged exposure or symptoms induced by successive pollen seasons.
- ∘
Patients with AR and asthma during the period of maximum exposure to the allergen.
- ∘
Poly-sensitized patients where AIT with mixtures of more than two allergenic extracts is considered to be used.
- ∘
- •
Sublingual immunotherapy treatment (SLIT) is indicated for the following cases:
- ∘
Mono- or poly-sensitized patients in whom AIT is considered mixing two allergenic extracts.
- ∘
Patients who have suffered systemic reactions with SCIT.
- ∘
Patients who have trouble adhering to SCIT or do not tolerate it.
- ∘
Other aspects to be considered before initiating AIT treatment include100(level of evidence level 1a, grade of recommendation A):
- •
Provocation test with the responsible allergens should be carried out, although it is not the usual practice in children.
- •
Identification of other triggering factors that might be involved in the symptoms.
- •
The stabilization and control of respiratory functions (essential in patients with asthma).
- •
Symptom response to the drug.
- •
Availability of standardized or high quality allergenic extracts.
- •
Socioeconomic factors, such as cost or the patient's occupation.
In patients with asthma, the AIT can be used before administering inhaled corticosteroids (ICS) for patients with mild allergic asthma and concomitant AR. AIT can also be used in patients using ICS alone or inhalers containing corticosteroids and leukotriene receptor antagonists and/or omalizumab if asthma symptoms are controlled. However, to reduce the risk of severe reactions, asthma symptoms must be controlled and forced expiratory volume in 1 second (FEV1) should be greater than 70% predicted at the time AIT is administered76(level of evidence 4, grade of recommendation C).
1.1.7Relative and absolute contraindicationsAIT is contraindicated in patients with conditions that increase the risk of severe systemic reactions related to treatment, such as those with severe or poorly controlled asthma and those with significant comorbidities such as cardiovascular diseases, cancers, immunodeficiency and autoimmune diseases98(level of evidence 4, grade of recommendation C). SCIT is contraindicated when the observation of the injection site or when the monitoring during the 30min after the injection is not possible98(level of evidence 4, grade of recommendation C).
If a systemic reaction occurs during pregnancy, severe foetal hypoxia may occur or may advance uterine contractions.107 Therefore, AIT must not be initiated during the first trimester of pregnancy (threat of abortion) or during the third (threat of preterm labour). If it became necessary to initiate AIT during this period, it must be done during the second trimester. However, immunotherapy can be maintained during pregnancy as long as the patient benefits from it and tolerates the injections108(level of evidence 4, grade of recommendation C). A prospective study evaluated 185 pregnancies with SLIT, including 24 women who started immunotherapy during pregnancy.109 The incidence of obstetric complications was lower than in the general population or in women who received drug therapy alone. Only 6% of patients showed local reactions and no systemic reactions with SLIT. The authors concluded that SLIT is safe during pregnancy and can be started in this period (level of evidence 4, grade of recommendation D). A recent review of the literature was conducted to evaluate the safety of initiation and continuation of AIT during pregnancy.110 Data analyzed showed no significant difference in the incidence of prematurity, hypertension/proteinuria, congenital malformations or perinatal deaths between the women continued on AIT (SCIT and SLIT) during pregnancy and controls.
Patients with problems of adherence to other forms of treatment are not likely to benefit from AIT treatment, since they will surely need frequent dose alterations, thereby increasing the chance of errors.111 AIT is also contraindicated in patients taking β-blockers and angiotensin-converting-enzyme inhibitors, since these agents can amplify the severity of the reaction, may mask the early signs of anaphylaxis and make the treatment for systemic reactions more difficult111–116(level of evidence 4, grade of recommendation C). There is no contraindication for AIT in patients treated with monoamine oxidase inhibitors, but caution is recommended with the use of epinephrine in patients treated with them117(level of evidence 4, grade of recommendation D).
Patients with severe and poorly controlled asthma have an increased risk of systemic reactions to immunotherapy than patients with stable and well-controlled asthma. AIT is also contraindicated for patients with angioedema.99
Historically, age and some diseases have been considered relative contraindications for AIT treatment. However, a survey conducted among members of the American Academy of Allergy, Asthma and Immunology (AAAAI) showed previous experience exists in the treatment of patients with certain diseases by SCIT.118 Based on this experience, it seems that SCIT treatment in patients with a history of cancer, which have undergone a transplant or are human immunodeficiency virus (HIV)-positive (but have not yet developed acquired immune deficiency syndrome [AIDS]) still poses no significant risk118. Although some physicians prefer not to alter the immune system of patients with autoimmune disorders, immune deficiency syndromes or cancer, there is no solid evidence that proves that AIT is harmful to these patients, as long as the risks and benefits of therapy are taken into consideration92(level of evidence 4, grade of recommendation D). It has been empirically recommended that in patients with controlled HIV infection (≥400CD4/ml) with no history of opportunistic infections or other pathology associated with AIDS and the absence of HIV viraemia, immunotherapy may be indicated after the mandatory consent of the patient.119,120
Regarding the age of the patient, the age limit for SCIT is no longer set at 5 years of age, although this is an area that still requires further study. With regards to treatment with SLIT, some data exist on children three years or older118 and on its continuation during pregnancy.109
Considerations in patients with atopic dermatitisThere is evidence that patients with atopic dermatitis, with clinically relevant sensitizations or allergies to airborne allergens can benefit from AIT.121 In a recent meta-analysis on the effect of immunotherapy on atopic dermatitis, including placebo-controlled, double-blind studies that assessed both SCIT and SLIT, the authors concluded that there is a moderate evidence of immunotherapy in atopic dermatitis122(level of evidence 1a, grade of recommendation B). However, this indication should be considered with caution as more studies are necessary.
Etiologic treatmentMechanisms of allergen-specific immunotherapyAIT inhibits the allergic early phase as well as the late response123,124 and it is characterized by decreases in the sensitivity of end organs and changes in the humoral and cellular responses to the administered allergens.76 Different immunological effector cells are responsible for allergic inflammation.125,126 The allergic disease is driven by the subset 2 of T-helper (Th) lymphocytes, which are characterized by the production of cytokines such as IL- 4, IL-5 and IL-13 among others. These cytokines are responsible for the effects on other cells involved in the allergic response (eosinophils, mast cells and basophils).127 Cellular and molecular events that take place during the course of AIT can be classified into different stages.
1.1.8Changes in humoral immunityWhen AIT is initiated, allergen-specific IgE levels usually show an initial increase and then a gradual decrease during the years of treatment. There is also an increase in allergen-specific IgG antibodies that may persist for many years after AIT is discontinued,128 not being predictive of the degree or duration of efficacy of immunotherapy.111 Levels of specific IgG1, IgG4 and IgA increase. None of these changes in antibody levels has been shown to correlate with clinical improvement.76 IgG4 is thought to act as “blocking” antibody. This immunoglobulin inhibit IgE-facilitated allergen uptake by dendritic cells and prevent IgE-mediated allergen activation of basophils and mast cells with the consequent inhibition of the release of inflammatory mediators.129 Thus, the blocking effects of IgG4 may have an important role in suppressing IgE-mediated T-cell activation. It is believed that allergen-specific IgG4 may reduce the sensitivity of antigen-presenting B-cells and therefore T-cells to allergens by competing with IgE.128 Because the production of IgE against normally harmless antigens causes allergic responses, the production of antigen specific IgG can antagonize and block the allergic inflammatory cascade resulting from antigen recognition by IgE. Therefore, the shift in balance between IgE and IgG4 may be essential to successful AIT.130
Specific IgA2 levels are also increased (although in a more modest way) after AIT,131 and secreted specific IgA seems to play a protective role at mucosal surfaces. The isotype IgA2 may also act as “blocking antibody” at the mucosal surface.
1.1.9Changes in cellular immunityThe induction of tolerance in peripheral T-cells is an essential step in AIT. Immunologic tolerance is defined as a sustained decrease in allergen-specific T-cell responsiveness. With continued immunotherapy, there is some waning of this response and it predominates the immune deviation from Th2 to Th1 cytokine response to the administered allergen.76 Thus, there are diminished levels of IL-4 and IL-5 and enhanced synthesis of interferon gamma (IFN-γ). Also, the presence of suppressive cytokines such as IL-10 and transforming growth factor beta (TGF-β) are important for controlling the allergic inflammatory response.132 Besides Th1 cells and cytokines orchestrating the suppression of allergic inflammation, a third subset of T-lymphocyte, referred as regulatory T-cell (Treg), plays a significant role for the development of a balanced Th2/Th1 profile with down-regulatory tone in allergic reaction.133
Atopic patients have a decrease in their Treg function. Studies of AIT with Hymenoptera venom, grass pollen extract134 and house dust mites131 demonstrate that there is a shift of CD4+ Th-cells from producing Th2 (IL-4, IL-5) to Th1 cytokines (IFN-γ, TGF-β, IL-10) following stimulation with allergen. Early production (soon after the beginning of AIT) of IL-10 and maintained levels of TGF-β are related to an efficacious AIT.
IL-10 is a potent immunosuppressive cytokine involved in tolerance. It reduces proinflammatory cytokine release from mast cells, eosinophils and T-cells; and elicits tolerance in T-cells by means of selective inhibition of the CD28 co-stimulatory pathway.135 It is produced by Treg, which also have the ability of producing TGF-β; another immunosuppressive cytokine that inhibits specific IgE and IgA production. It also suppresses Th1 and Th2 cells.136 AIT-induced IL-10 production is not only limited to T-cells, but also produced by B-cells, monocytes and macrophages. Consequently, lymphoproliferative responses to allergens are reduced after immunotherapy.137
There are different types of Treg with characteristic phenotypes and mechanisms of action. Among these Treg, the natural subset, expressing CD4 and CD5 and the transcription factor fox p 3 (FOXP3+CD4+CD5+), has been the most studied in the last years. The expression of the transcription factor FOXP3 is required for natural Treg function, and Treg development and expansion is dependent on TGF-β. Other subtypes of Treg, such as Tr1 and Tr3, can be induced by different stimuli.111 Treg are considered the “master regulators” of immune homeostasis.138 They play a major role in maintaining immune self-tolerance in the periphery and protect against excessive activation and disease. They have an important role in inducing tolerance in allergen-specific T-cells in healthy and in allergic subjects following AIT.111
The last phase in a patient submitted for AIT after several months is the decrease in tissue mast cells and eosinophils, and the release of their mediators. It is accompanied by a decrease in type I skin test reactivity. Multiple cell types in the blood and affected organs show changes and contribute to allergen-specific immune tolerance development.139
The oral mucosa is a natural site of immune tolerance.138 SLIT has been shown to induce long-term remission after discontinuation and may prevent new sensitizations, features that are consistent with the induction of tolerance. Moreover, additional local mechanisms in the oral mucosa and/or regional lymph nodes are likely important. Proteins are rather captured by professional antigen-presenting cells (APC) within 15–30min, which will subsequently migrate to draining cervical submaxillary lymph nodes within 12–24h.140 The presence of Langerhans cells and monocytes, particularly in the vestibular region, capable of producing IL-10 and TGF-β, are major contributors to the maintenance of tolerance.141 Abundant FOXP3+ Treg were also detected in lingual and palatine tonsils.142 On the other hand, only few proinflammatory cells (mast cells and eosinophils) are found in oral tissues and they are located in the lower layers.143 In this context, most allergens are likely captured by tolerogenic dendritic cells in the upper layers of oral tissues prior to reaching proinflammatory mast cells, thus explaining the excellent safety profile of the sublingual route.144 All these items make the sublingual route an efficient route for AIT.
The initial rise in IgE, IgG1 and IgG4 responses has been demonstrated during SLIT. This administration route shifts allergic-specific CD4+ T-cells responses from Th2 to Th1, with the stimulation of IFN-γ-producing T lymphocytes.145,146 In addition, SLIT also induces Treg, which, as we already have described, play a central role in inhibiting effector mechanisms associated with allergic inflammation.147
Regulatory dendritic cell markers, such as C1Q, are upregulated in peripheral blood mononuclear cells of patients with grass pollen allergy exhibiting clinical benefit during AIT. A combination of 5 markers predominantly expressed by blood dendritic cells (i.e., C1Q and CD141) or shared with lymphoid cells (i.e., GATA3, RIPK4 and FcRIIIA) reflecting changes in the balance of regulatory/proallergic responses in peripheral blood can be used as early as after two months to monitor the early onset of AIT efficacy.148
Types of AIT1.1.10Types of extractsAllergenic extracts used in SCIT can be unmodified (the final formulation of those allergens present in an original state, without undergoing any further modification) or physical, chemical or physicochemically modified in order to increase their efficacy and/or safety.149
Aqueous extracts: Extracts are lyophilized to be lately prepared in phenolated saline solution or not, usually with mannitol and glycerol.150,151
Depot extracts (physically modified extracts): Allergens are combined with substances such as aluminium hydroxide, calcium phosphate, tyrosine or liposomes with the aim of increasing efficiency and reducing adverse effects.150,152–156 Chemically modified extracts: Extracts are modified by treatment with formaldehyde, glutaraldehyde or alginates or by depigmentation. As a result of these modifications allergenicity is reduced or eliminated, while immunogenicity or the ability to modulate the immune system is maintained or increased.157,158
- •
Combined modification of the extracts: Extracts are physically and chemically modified (modified with formaldehyde and absorbed in aluminium hydroxide; modified with glutaraldehyde and absorbed in tyrosine; polymerized with glutaraldehyde and absorbed in hydroxide aluminium).157,159
SCIT is the traditional administration route of AIT and is now considered the gold standard.160 However, the disadvantage of the frequent visits necessary for the administration of the treatment, the discomfort associated with the injections and the possibility of adverse reactions has motivated research of alternative ways of administrating effective doses of immunotherapy.
In the last decade, several SLIT products administered as lyophilized tablets have been commercialized. They have met the highest requirements in terms of evidence-based medicine regarding efficacy, tolerability and pharmacopoeia, and can be equated with any pharmaceutical product used so far. Grazax® (ALK Abelló) contains a standardized allergen extract of P. pratense at a dose of 75,000 SQ-T oral lyophilisate (this dose is equivalent to 15μg of major allergen Phl p 5).161 Oralair® (Stallergenes) contains a natural standardized allergen extract of 5 grass pollens (Lolium perenne, Poa pratensis, Anthoxanthum odoratum, P. pratense and Dactylis glomerata) at a dose of 300 index of reactivity (IR) (roughly equivalent to 25μg of the major allergen Phl p 5).162 The overall evaluation of 21 published meta-analyses for SLIT would highlight the heterogeneity of clinical trials, which present large variations in the population studied, in inclusion criteria and in the score systems used for assessment of efficacy regarding symptoms and pharmacotherapy.163
It would also be useful to have standardized allergen doses administered, since considering them, these range from 3 to 500 times the cumulative dose used for SCIT. A dose-response relation has been published and for the commercialization of the tablets the dosing of both products has been studied.164 After the respective tests of safety and dose, they have both shown efficacy in the short and long term in children and adults, and a detailed safety profile has been designed.165–167 Nevertheless, all these facts cannot be extrapolated to each product commercialized.
1.1.12MixturesThe efficacy of AIT in mono-sensitized patients has been proven in both children and adults and with both routes of administration, SCIT and SLIT.168–170 There is no consensus in the therapeutic approach of poly-sensitized subjects. Thus, while mono-AIT is preferred in European countries, the usage of more than 8 allergens in the same shot is common in the USA.171 In this sense, the European Medicine Agency (EMA) recommends allergists to restrict the mixture of non-related allergens to a minimum and gives advice not to mix seasonal and perennial allergens, or allergens with proteolitic activity without justification.172 Having taken all of these into account, it is of the utmost importance to precisely define the diagnosis before prescribing AIT.20
A Spanish consensus study was published and some criteria have been established to improve AIT prescription in poly-sensitized patients helping allergists to better identify relevant allergens in this kind of patients and to improve selection of AIT in each case.173
1.1.13Other associated therapies: omalizumabThe use of the anti-IgE omalizumab to increase the efficacy and safety of AIT has been described in several studies with pollens, Hymenoptera venoms and food174–183(level of evidence 1a, grade of recommendation A).
Dosage guidelines/treatment regimenAIT is administered in two phases: the initial build-up phase, when the dose and concentration of the extract is increased and the maintenance phase with fixed, optimal and maximum dose during intervals of between 4 and 6 weeks. At present, there are modified extracts where the starting dose is very closely administered to the maintenance dose (level of evidence 4, grade of recommendation C), so there is virtually no starting dose and should not be confused with rush or ultrarush doses.
1.1.14Initial dose and increment doseThis phase consist of the administration of increasing doses at intervals until reaching the maximum or optimal dose. There are several ways: (1) conventional treatment regimens begin with very diluted allergen doses at weekly intervals, for about 4–16 weeks depending on the formulation of the extract. The allergen starting dose is usually 5000–10,000 less than the maintenance dose, where no efficacy is assumed. It confers good safety and the systemic reactions rate is almost insignificant; and (2) cluster treatment regimens are generally administered in two doses per visit at weekly intervals, and the optimal maintenance dose is reached in 2–4 weeks. The average concentration (dilution 1/10vol.) vial or the higher concentration vials are used directly to begin treatment.
Multiple studies have demonstrated that the incidence of systemic reactions is similar in both treatment regimens, with all extracts (native, modified, SCIT and SLIT) and with all allergens. At present, the reasons for not using the cluster treatment regimen are the accessibility to AIT units, work load and experience of the prescribing person, and patient's available time.184–190 The fast-acting treatment regimens (rush and ultrarush) are very accelerated treatment schedules where all the starting doses are administered in 1 to 6 days reaching the maximum-optimal dose in 1 week. These treatment regimens have been tested with SCIT191 using both modified and native (in the case of Hymenoptera venom) and with SLIT.192 The safety of these regimens varies from one study to another, although most authors agree that it is necessary to be administered with caution by experienced physicians in AIT with sufficient infrastructure193–195(level of evidence 1b, grade of recommendation A).
1.1.15Maintenance treatment regimenThe maintenance treatment regimen for SCIT consists of a single fixed dose, which should coincide with the maximum recommended or tolerated dose at 4–6 week intervals with a minimum duration of three years and maximum duration of 5 years196,197(level of evidence 1b, grade of recommendation A).
The technical data sheet of the product determines the dosing interval, although in some SCIT studies with Hymenoptera venom the patients tolerated intervals of up to 3–6 months with the same efficacy and safety.198–202 No studies have shown that increasing the maintenance interval beyond 6 weeks has an effect on the efficacy with other extracts administered subcutaneously.
The SLIT maintenance treatment regimens vary in intervals ranging from one dose daily to three doses weekly with the same safety and effectiveness, administered at home. Lyophilized tablets should be administered daily203(level of evidence 4, grade of recommendation D).
Depending on the allergen, the treatment regimens may be preseasonal (begins and ends before the start of the pollen season), coseasonal (begins and ends during the pollen season), precoseasonal (starts before the pollen season and continues until its end) or perennial (throughout the year).
1.1.16Precoseasonal versus preseasonal regimensThe current trend is to carry out SLIT precoseasonal treatment regimens because longer therapies favour the non-compliance of the patients. In an analysis of 41 placebo-controlled studies with pollen SLIT, three studies had used a preseasonal; three a coseasonal; 8 a perennial and the remaining 27 precoseasonal regimens.204 In addition, an open, placebo-controlled study in children with rhinoconjunctivitis due to grass pollen compared the clinical efficacy and safety of a preseasonal and precoseasonal regimen (the latter with two different maintenance dose).205 Findings showed that a precoseasonal regimen was significantly more effective, especially with the higher maintenance doses. Precoseasonal regimen com is the best choice to reach efficacy with SLIT from the first pollen season (particularly with grass pollen, beginning at least 8 weeks before the pollen season and continuing until the end of it) (level of evidence 2b, grade of recommendation B).
1.1.17Precoseasonal versus perennial regimensThere are few publications comparing precoseasonal and perennials regimens, as well as their influence on the efficacy throughout several pollen seasons. A placebo-controlled study compared the clinical efficacy of preseasonal and perennial SLIT regimens during two years.206 Both protocols were effective compared to placebo, showing a similar efficacy in reducing symptoms and medication use, as well as other secondary parameters such as monitoring peak expiratory flow (PEF), FEV, PD20 in the methacholine challenge test or the determination of FENO. The precoseasonal therapy was more effective in reducing nasal symptoms, with no significant differences in bronchial and ocular symptoms.
More recent well-designed studies have shown that SLIT based tablets 300 IR with 5 grass pollen in a precoseasonal regimen administered during three pollen seasons, is effective in reducing symptoms and rescue medication from the first pollen season analyzed.165,196
EfficacySCIT and SLIT have shown to be effective and safe for the treatment of AR.20 The ARIA guidelines consider AIT for both adults and children with moderate to severe, persistent or intermittent AR.207–209 In addition, its efficacy is maintained long term even after discontinuation of immunotherapy210 (level of evidence 1b, grade of recommendation A). The potential role of AIT in preventing asthma is specifically stated in this guideline, and recommendations regarding the management of AR and asthma when they coexist in the same patient are given.
According to ARIA, adults with AR but without asthma may use either SCIT or SLIT to treat pollen or house dust mite allergy. However, ARIA places a high value only on SCIT for reduction of symptoms and potential prevention of the development of asthma in children. Thus, SLIT may be recommended for adults with rhinitis caused by pollen or house dust mite, but only pollen SLIT should be recommended in children until the efficacy of house dust mite SLIT is proven in controlled clinical trials in children.
This different approach to SLIT depending on the allergen is difficult to understand. A systematic review of SLIT published in 2011 revealed a significant improvement in symptoms (standardized mean difference [SMD], −0.49; 95% CI, −0.64 to −0.34) and medication scores (SMD, −0.32; 95% CI, −0.43 to −0.21) after analysing 49 double-blinded, placebo-controlled, high quality studies.151 No differences were observed depending on the allergen or the age of patients (15 of these studies had been performed in children). Perhaps the cautious recommendation given to children with house dust mite allergy is due to the small number of studies using this allergen.
As the Global Initiative for Asthma (GINA) suggests, the main limitation for using AIT in the treatment of AR is the risk of adverse side effects.211 However, many clinical trials and daily clinical practice rule out this supposedly high rate of serious side effects due to AIT.212 It must be remembered that this evidence cannot be extrapolated to each product and marketed allergen.
1.1.18Variables to be measured at the beginning, during and after treatmentDepending on the duration of the studies, the efficacy of AIT can be divided into short-term, sustained and long-term effect. We will refer to long-term efficacy as “the prolonged clinically relevant benefit that persists after stopping the treatment”.213 In 1988, Mosbech and Osterballe first demonstrated that the effect of AIT could last after termination of treatment.214 The median symptoms for the sixth season were more than 33% of the pre-treatment values in spite of a 10% higher pollen exposure. Durham et al. have shown a long-term clinical efficacy after providing a prolonged clinical remission of symptoms for at least three years following discontinuation of SCIT with a grass-pollen extract.215
In an open study performed by Des Roches et al., in both child and adult asthmatic patients treated with a standardized D. pteronyssinus extract, it was shown that less than three years of treatment is associated with a relapse of asthma symptoms within the first years after discontinuation.216 In a retrospective survey, asthmatic adults allergic to house dust mite or to both house dust mite and grass pollen who had received SCIT during childhood were compared to 42 asthmatic adults who has been treated with conventional treatment.169 For this purpose, patients and controls were assessed by standardized questionnaire and lung function tests. Even though it is not a prospective blind study, it resembles clinical practice and it is noteworthy that 9 years after finishing SCIT, asthma symptoms were significantly reduced in the AIT group.
During a prospective 5-year follow-up, symptom and medication scores were recorded, and a significant improvement was evident in SCIT group with no need of ICS.217,218 The study was performed in both children and adults. Authors showed a small margin of benefit after two more years of treatment for AR symptoms. In a prospectively designed, open, parallel-group controlled study, Di Renzo et al. treated children with house dust mite-allergic asthma for 4–5 years with either house dust mite-SLIT plus pharmacological treatment (n=35) or pharmacological treatment alone (control group, n=25).22 Clinical evaluation (symptom and medication scores) at baseline and 5 and 10 years later showed a significant improvement in those patients who had received SLIT (p<0.001), but not in the control group. A similar open study was performed in children with house dust mite-SLIT, showing a steroid-sparing effect that persists for at least 6 months after discontinuation of SLIT in the active group.219
1.1.19Prevention to progression into asthma and to new sensitizationsThe Preventive Allergy Treatment (PAT) study is a European multicentre study designed to find out if three years of SCIT could prevent children from developing asthma. Two hundred and five children (6–14 years) suffering from seasonal AR due to birch and/or grass pollen were recruited. After three years of treatment, a significantly higher number of children in the control group developed asthma (odds ratio, 2.52; 95% CI, 1.3–5.1; p<0.05), favouring the hypothesis that SCIT can prevent the development of asthma.220–222 Two and 7 years after termination of the treatment (5- and 10-year follow-ups), the preventive effect of SCIT persisted and was even greater, reaching an odds ratio of 4.6 (95% CI, 1.5–13.7) in the latter analysis of the 117 available patients without asthma before the start of the study.220,222 A similar approach was made by Eng et al., using a grass-pollen allergoid and proving a similar benefit 12 years after discontinuation of SCIT in a small group of 14 children compared to a similar group of non-treated grass-allergic children.23,223
The Paediatric Investigation Plan of the EMA was born to support the idea that AIT should only be given to children if a disease-modifying effect is demonstrated after three years of treatment, followed by two more years without it. In this context, a European multicentre initiative aimed to investigate the preventive effect of marketed grass tablets (SLIT). Currently, the GAP study (5-year-term double-blind, placebo-controlled) is being carried out to investigate the preventive effect of AIT for developing asthma in children suffering AR.159
SafetyAIT has proven to be safe when it is administered properly100,107,224,225 (level of evidence 2a, grade of recommendation B); this safety varies depending on many factors. The wide variability in published studies was the reason why the World Allergy Organization (WAO) established a classification of systemic reactions in 2010.226 This new classification replaces the previous one. The WAO includes new concepts such as the degree of severity of symptoms and clarifies the Sampson et al. definition of traditional anaphylaxis.227 The WAO specifies that if the symptoms of a single organ are severe (airway angioedema or bronchospasm) it is sufficient to indicate the administration of adrenaline.
With this new classification, different SCIT studies estimate systemic reactions incidences ranging 0.1–1.56% per administered dose.20,116,191,228–230 The American College of Allergy, Asthma and Immunology (ACAAI) observed 0.1% of systemic reactions per dose in a study that included more than 8 million doses administered; 74% of these systemic reactions where grade 1 and 23% grade 2.231 Anaphylactic reactions were estimated at three per million doses and in patients with risk factors (bronchial asthma and initiation phase) and one per million in patients without risk factors.232 Later, the same group with 23 million doses reported a systemic reactions rate similar to the previous one and only one case of fatal reaction.233 Weber et al., with a total of 12,895 patients (2441 paediatric), reported a systemic reactions incidence of 0.5% per dose in adults and 1.2% in children.228 No grade 5 reactions have been reported in children. Most systemic reactions occur in the first 30min of administration and in the start-up phase.
SLIT is safer than SCIT. In fact, most treatments are administered at home. The most common reactions with SLIT are local (oral itching and mild oedema) and usually occur in the first days of administration. Systematic reviews on SLIT with more than 5131 patients (1814 children) reported frequent local reactions.151,234–236 Up to 79% of patients experienced a local reaction, while systemic reactions only occurred in 0.056% of the doses administered. Di Rienzo et al. report 1.5% of local reactions in 128 children22 while Agostinis et al. report 32%.237 Due to the high variability of studies, the WAO also reached an international consensus on the local reactions grading system for SLIT in 2013 (Table 4).237,238 Some authors suggest that lesions in the oral mucosa may be a risk factor in SLIT.
Local reactions grading system for SLIT.
| Symptom/sign | |
|---|---|
| Pruritus/swelling of mouth, tongue, or lip; throat irritation, nausea, abdominal pain, vomiting, diarrhoea, heartburn, or uvular oedema | |
| Grade 1: Mild | • Not troublesome • No symptomatic treatment required • No discontinuation of SLIT because of local side effects |
| Grade 2: Moderate | • Troublesome • Requires symptomatic treatment • Nos discontinuation of SLIT because of local side effects |
| Grade 3: Severe | • Troublesome • Requires symptomatic treatment • SLIT discontinued because of local side effects |
| Unknown severity | • Treatment is discontinued, but there is no subjective, objective or both description of severity from the patient/physician |
SLIT: sublingual immunotherapy treatment.
There are few studies comparing the incidence of systemic reactions in children and in adults and it is not possible to specify in which group it is more frequent. Weber et al. report a systemic reactions incidence of 1.2% per dose in children compared to 0.5% per dose in adults.228 However, other studies did not confirm these findings. Roberts et al. showed an incidence of similar systemic reactions to that found in studies with adults in a randomized double-blind study carried out in 122 children aged 3–16 years old with SCIT.239 Another study showed 0.01% of systemic reactions per dose in 239 children aged 1–5 years old (6689 doses) with SCIT.240 There is greater difficulty in the early diagnosis of possible systemic reactions, but not a greater risk.241 There is no contraindication demonstrated with sufficient scientific evidence that showed that AIT is less safe in children under 5 years239–241 (level of evidence 4, grade of recommendation C).
Bronchial asthmaUncontrolled bronchial asthma is a systemic reaction risk factor in SCIT.242,243 Bernstein et al. showed an odds ratio of 12.1 (95% CI, 2.6–61.0; p<0.001) in patients with partially controlled asthma and 37.4 in those with uncontrolled asthma (95% CI, 5.7–251.1; p<0.001).244 Another study of 21,022 doses administered showed a total of 131 anaphylactic reactions as well as a strong association between uncontrolled asthma and the risk of serious systemic reactions.245 Schiappoli et al. report that in a total of 60,875 doses administered, systemic reactions occurs more frequently in patients with asthma than in those with rhinitis or rhinoconjunctivitis alone (4.1% versus 1.1%).246 It is recommended with evidence B to administer AIT to patients with well-controlled asthma and to carry out a spirometry or PEF before dose,76,247 establishing the premise that AIT should not be initiated in patients with persistent uncontrolled asthma due to serious risk of systemic reactions.
Previous local reactionsPrevious local reactions do not increase the risk of systemic reactions, therefore no dose adjustment is recommended.107,247–249 However there is no consensus in the grading of local reactions, defined as oedema, pruritus and erythema at the injection site. Some authors describe the definition of large local reactions as redness and swelling measuring 25mm or greater around the site of injection,250 others as larger than the diameter of one dollar251 and others like the palm of the patient's hand.252 In consecutive studies in patients with systemic reactions, one with dose adjustment and another without it, Tankersley et al. observe neither a significant differences between the two groups nor an increase in the frequency of systemic reactions.253–255 The REPEAT study with 9679 doses administered confirms these findings.256 However, other studies defined large local reactions of more than 25mm as a risk factor for systemic reactions.113,247 Roy et al., with 661 patients (1,108,621 doses), observed 4 times more large local reactions in patients with systemic reactions than in those who have never showed systemic reactions (35.2% versus 8.9%; p<0.001).250
In conclusion, it appears that local reactions under 25mm are not predictors of systemic reactions, so they do not need dose adjustment. More studies are needed to clarify whether large local reactions are risk factors or not. Many authors suggest that premedication with antihistamines or montelukast decrease the frequency of local reactions.255–257
Systemic reactions to previous dosesBecause most systemic reactions occur in the first 30min, all patients should remain under observation during this time after administration of the dose116 (level of evidence 4, grade of recommendation C). In daily practice, the dose is adjusted after a systemic reaction due to the risk of recurrence. However, scientific evidence is not clear on this point. When a systemic reaction occurs in a dose increase, the general practice is to return to the previous well-tolerated dose, but in the maintenance phase there are no clear recommendations.
In the study by Weber et al., the incidence of systemic reactions recurrence after dose adjustment or non-adjustment is compared, showing an increase in the rate of recurrence in the group that did not adjust, but only in the start-up phase and not in the maintenance one.228 These results are corroborated by the American Task Force, who states that there is insufficient evidence to adjust the dose maintenance phase after a systemic reaction76 (level of evidence 5, grade of recommendation D).
Another question is whether premedication with antihistamines reduces or not the frequency of systemic reactions. The above mentioned studies present lower systemic reaction rates in patients treated with antihistamines, but only in cluster and rush regimens, in AIT with Hymenoptera venom and pollen extracts, and not in the maintenance phase255–257 (level of evidence 1b, grade of recommendation A).
Poly-sensitizationThe case of poly-sensitized patients is not sufficiently documented. A study analyzed the administration of two extracts in poly-sensitized patients using SCIT with different allergens (n=95) compared with administration of only one (n=52).258 Local reactions increased only in the start-up phase (1.5% in the double dose administration versus 0.7% in the single administration), but systemic reactions increased (0.27% versus 0.23% dose) neither in the start-up nor in the maintenance phase.258 They concluded that a double administration poses no greater risk of systemic reactions. Others report higher systemic reactions rates, which indicate that more studies on the safety of double or multiple AIT are needed.259
1.1.21Factors dependent on the extractOnly registered standardized allergen extracts should be administered. The modified extracts have lower systemic reactions rates than those with native allergen extracts.191,260,261 Likewise, the sublingual route formulated with native allergens is the safest, despite local reactions262 (level of evidence 1b, grade of recommendation A).
With regards to the composition of the extract, no allergen seems less safe than another. Years ago, some guidelines indicated that the AIT with fungi in children produced more reactions than other extracts. Cantani et al. do not describe any systemic reaction in a prospective study of 39 children using Alternaria AIT.263 Other studies support this finding189,264 (level of evidence 2b, grade of recommendation B).
1.1.22Factors dependent on the treatment regimenAccelerated regimens (cluster, rush and ultrarush) versus conventional SCIT therapyAs discussed above, the majority of systemic reactions in SCIT occur in the initial phase. Numerous studies have shown that cluster treatment regimens are safe and have similar systemic reactions incidence rates than those of conventional treatment regimens.187–189,265 Nieto et al. observed 0.1% systemic reactions per dose in a study carried out with 1245 Dermatophagoides sp. extract doses administered in cluster regimen to children, a percentage similar to those found in studies with millions of conventional starting doses.260 Other studies in the paediatric population do not show higher rates of systemic reactions when compared with conventional treatment regimens or with cluster regimens in adults, even in cohorts of asthmatic children186,266,267 (level of evidence 1b, grade of recommendation A).
As for the much accelerated regimens (rush and ultrarush), it seems that they present greater systemic reactions than cluster and conventional regimens with aeroallergen extracts, and they decrease after administering premedication with an antihistamine. Cardona et al. administered polymerized (modified) Dermatophagoides sp. ultrarush regimens (1 day) to 575 patients (aged 1–83 years).191 They observed 0.11% of systemic reactions per dose; all were grade 1–2 with 83.1% of patients being premedicated with an antihistamine. Paediatric age and bronchial asthma were identified as risk factors for the incidence of SR. There is more experience with Hymenoptera extracts in ultrarush regimens showing similar incidences of systemic reactions than with other regimens.268–271
Accelerated SLIT schedulesAs mentioned above, the sublingual route is safe but frequently presents local reactions in the starting phase. Neither the rush nor the ultrarush regimens seem to increase the incidence of local reactions. In a study of 679 patients with an ultrarush regimen where the dose was administered every 5min (25min accumulative doses from 4.7 to 525μg of major allergens), 17.96% of local reactions and 0.2% of grade 2 systemic reactions were observed.272 In another study of 218 patients (122 minors under 15 years of age), Dermatophagoides sp. extract in a rush regimen (dose every 30min) was administered.192 Some type of reaction was developed by 12.4% of patients (59.3% boys), 74.9% of them were local reactions (oral and gastrointestinal) and the rest were grade 1–2 systemic reactions. The treatment regimen was slowed in only two patients. This study indicates that asthmatic patients do not have a higher rate of adverse drug reactions, unlike in SCIT. Concerning sublingual tablets, in a study of 175 children, 75% had some local reactions,273 but other authors with similar samples observed up to 87% of local reactions per child.274 Nevertheless, they all conclude that local reactions are temporary and the incidence of abandoning treatment with sublingual tablets ranges 2–5% of the children (level of evidence 1b, grade of recommendation A).
1.1.23Factors dependent on the personnel and place of administrationEvidence A (low risk for systemic reactions) is established when trained personnel administered AIT in a suitable location76 (level of evidence 1a, grade of recommendation A). Administration errors and possible late diagnosis of anaphylaxis have been identified as risk factors.45,236,275,276 The American Task Force described the recommended medication and team needed for the administration of the treatment for possible anaphylaxis.76 AIT in patients with bronchial asthma, previous systemic reactions and delays in the doses should be treated in immunotherapy units as well as all the cluster, rush and ultrarush accelerated regimens.
1.1.24Dose adjustmentsThe aim of dose adjustments is to ensure patient safety, meaning that a systemic reaction must be avoided. None of following cases is backed by good scientific evidence. Dose modifications are applied when a systemic reaction occurred in starting phase, in cases of repeated local reactions and when the treatment is delayed. Premedication is administered to evaluate cases of repeated local reactions, when systemic reactions occurs in starting phase and in both rush and ultrarush regimens.
Dose adjustments in late dosesInitial phase: In case of delays in the starting phase, the usual practice is to repeat the previously tolerated dose, although this may depend on the delay time. There is no consensus on the dosage. Weber et al. proposed an adjustment model starting from a two week delay (previous dose 2–4 weeks before) and subsequently lowering one dose for each week of delay (up to 8 weeks maximum).228 This model presents higher systemic reactions rates than the ones without delays. In a pilot study, 16 dose adjustment due to delay protocols were evaluated, all of them presented highly variability.277 Most of them have been calculated considering the volume (dose) and others by decreasing the percentage (American practice).76 A similar model to Weber et al. is proposed, in which one dose is reduced for each week of delay starting from 3 weeks. In Spain and Europe, the dose is adjusted according to the guidelines established by the manufacturer, but a general consensus on this matter does not exist.
Maintenance phase: Several studies have reported that adjustments are made starting from an 8 week delay, with a decrease of the previously tolerated dose depending on the delay interval, varying from one to two weeks76,229,277,278 (level of evidence 1a, grade of recommendation A).
Dose adjustments for a new vialNo recommendations exist for the adjustments of the dose in the case of a new vial is used. An increased incidence of systemic reactions has not been observed with standardized extracts76,229 (level of evidence 1b, grade of recommendation A).
Dose adjustments during the pollen seasonStudies carried out two decades ago recommended dose adjustment in SCIT with pollen extracts during the pollen season. Currently there is no evidence of this claim. In one study, although outdated, with 346,251 doses administered, no correlation was found between the levels of pollen and increased systemic reactions.279 Other studies with very large sample sizes corroborated these findings.280 This theory may be supported because the increase in systemic reactions observed during the pollen season worsens asthma symptoms. This factor causes confusion, since an increased risk does not exist in well controlled patients. The current recommendation states that dose adjustment is not necessary (level of evidence 3a, grade of recommendation C).
Immunotherapy adherenceSCIT and SLIT are effective treatments of AR and asthma, but high levels of compliance and persistence are crucial to achieve the desired clinical and immunological improvement. Adherence is defined as “the extent to which the patient's behaviour matches the agreed recommendations from the prescriber”.281 It involves not only acceptance and understanding of the therapy recommended, but also the treatment intake over the recommended period of time.282 Due to the long duration of immunotherapy, there is a problem with full adherence to the therapy and non-adherence contributes to poor clinical outcomes. Although an interaction of factors related to both the physician and the patient influence the adherence to a treatment, effective communication between these two parties and the simplicity of the regimen are of great importance.283
With regards to SCIT, published adherence rates vary widely, ranging from 13%284 to 89%.285 Many studies cite inconvenience as the primary reason for discontinuing SCIT.285,286 Other common reasons include unaffordable cost,285 adverse systemic reactions and medical comorbidities. Some patients discontinue the treatment because their symptoms resolve; on the other hand, others discontinue AIT because of ineffectiveness.285
Published adherence rates with SLIT vary between 30% over two years287 and 85% over three years.288 Reasons for discontinuing are local reactions (oral cavity itching and swelling), perceived ineffectiveness, administration difficulties, unaffordable cost, inconvenience and comorbidities.285–292 Adequate education of patients and optimization of administration schedules, with careful balancing between dose effectiveness and cost, are the factors most likely to achieve further compliance with AIT.293
The future of immunotherapyResearch on AIT is constantly changing; new information regarding molecular biochemistry and allergens and their epitopes have launched numerous research studies in order to improve efficacy in the shortest possible time, while at the same time preserving safety. The advances are heading in several directions: (1) modification of the extract (recombinants, peptides, chimeric, etc.); (2) Th1 immunopotentiating adjuvants fused or non-fused to recombinant allergens; (3) new vectors; (4) new administration routes such as epicutaneous (EPIT), intradermal and intralymphatic (ILIT).294–297 Although all studies with animals were reviewed since they provide a great beginning for new avenues of research for AIT, finally they were not included in this section because they do not provide any clinical insight that is the main objective of this review.
AIT with recombinant allergensThe use of recombinant allergens combined with the molecular diagnosis is an important step in maximizing the specificity of the extract. Currently, various allergen types are being tested: native (wild type allergen), recombinant allergens per se or modified and chimeric peptides and proteins.
The studies with a single purified major allergen extract (unmodified) show positive results in efficacy and safety with P. pratense pollen.298 Studies carried out by Pauli et al., in which complete birch pollen extracts were compared with unmodified protein (nBet v 1) and recombinant (rBet v 1) extracts, obtained better clinical results with the purified proteins than with the complete extracts.299
Another approach to AIT consists of modified recombinant allergens, which makes them hypoallergenic but with immunoreactivity. Niederberger et al. used a Bet v 1 extract fragmented into non-allergenic parts (no IgE binding) that retained the ability to interact with T-cells.300 Using the same idea, but fusing major allergen peptides, studies with Phl p 1, Ole e 1 and Alt a 1 extracts are being carried out.301–303
Combined allergens with adjuvantsThe discovery of immunomodulating adjuvants provides the possibility to directly activate the Th1 environment, thereby blocking the Th2. This results in the decrease of the Th2 cytokines (IL-4, IL-5 and IL-13) and in the activity of the effector cells, while potentiating the Th1 environment (IL-10, TGF-β and IFN-γ) acting through the Toll-like receptors (TLR).304,305 The TLR4 and TLR9 subclasses are the most studied.306 The fusion of native or recombinant allergens bound to a bacterial (CpG) or viral sequence would directly activate the Th1 route through the TLR triggering a Th1 response specific to the allergen.307–309 In a placebo-controlled study, 6 subcutaneous injections of purified Amb a 1 combined with CpG DNA sequence are administered, which targeted at a significant increase in Th1 cytokines and in a significant improvement of symptoms with no adverse reaction.310 Based on this data, several phase II clinical trials were initiated. One of them administered the same extract in 6 injections with an increasing dose range and a preseasonal weekly schedule to 28 allergic adults.311 A follow up during two seasons was carried out aiming for a significant increase of Th1 cytokines with a decrease of Th2 cytokines in nasal biopsies.312,313
Monophosphoryl lipid A (MPL) is derived from the lipopolysaccharides of Gram-negative bacteria. This immunomodulating adjuvant is being tested in a multitude of diseases such as Hepatitis B, Herpes simplex, Malaria and Alzheimer's, among others. The MPL is a potent Th1 stimulator via the TLR9 activation. A study carried out in 141 patients allergic to grass pollen analyzed the efficacy of an allergy vaccine containing MPL. Patients were randomized to receive grass pollen allergens absorbed in thyroxine containing MPL and placebo (tyrosine alone). This study showed a significant improvement in nasal and conjunctival scores, as well as a decrease in medication in the MPL category, with a short treatment schedule of 4 pre-seasonal injections.314 Other study of over 1000 patients who were administered the same conjugate obtained good clinical and immunological results.261
The virus-like particles are proteins from the virus capsid (Porcine parvovirus, Norwalk virus and papillomavirus) assembled with recombinant antigens such as Der p 1, Fel d 1 and Phl p 1. These combinations have already been tested in animal and human models with good results. Kündig et al. initiated a phase I study in 26 healthy volunteers with a conjugate of Der p 1 and a virus-like particle (ρβDer p 1) in a single dose.315 Their aim was a rapid increase in IgG1 and IgG3 4 weeks after one dose. Later, the same group published the phase I/IIa study with 20 allergic patients showing a significant reduction in clinical scores (asthma and rhinitis) two weeks after administration. These effects lasted up to 36 weeks after.316 Publications with recombinant Fel d 1317,318 and Phl p 1319 extracts combined with rhinovirus particles show potent responses in specific IgG4 measures and other parameters. In 2011, Klimek et al. published a phase IIb study with 299 patients allergic to mites.320 Participants are administered a conjugate of a virus-like particle with D. pteronyssinus extract (CYT003-QbG10), comparing two different doses with placebo. They observed an improvement in disease symptoms and reduced medication in patients treated with the highest dose of the conjugate.
Studies are also being carried for SLIT with bacterial adjuvants like Lactobacillus reuteri and Lactobacillus casei, which are described as potent activators for the production of IL-10 through Treg.321 Other adjuvants studied are detoxified bacterial toxins of Vibrio cholerae, Escherichia coli and Klebsiella pneumoniae combined with ovalbumin, Fel d 1 and Bet v 1 through TLR2.322 In addition, MPL in SLIT is being assessed in phase I/II studies with allergens from grass pollen showing negative nasal provocation after two months of administration. Phase II/III studies are currently underway.323
SCIT and SLIT vectorsMucoadhesive polymer microspheres (maltodextrin, chitosan, lactic acid, etc.), which are put together with a controlled sustained release of allergen pellet, amplify and facilitate the APC action.312,324 Basomba et al. conducted a double-blind, placebo-controlled study with Dermatophagoides sp. extract encapsulated in liposomes administered subcutaneously showing significant differences in the active group in terms of medication and provocation with no adverse reactions.325
Other AIT routes: epicutaneous and intralympathicDue to the high presence of APC in the skin, this area is an attractive organ for applying allergens with relatively few side effects.326,327 The first randomized double-blind placebo-controlled study was published in 2009 where 12 patients allergic to grass pollen were administered skin patches.328 A 70% improvement was observed in clinical scores in the active group versus 20% in placebo group. Systemic reactions were not observed, but local reactions were observed in the form of eczema. Later, the same group developed the phase I/IIa study with 132 patients allergic to grass pollen.275 Symptoms were reduced by 30% in the first year and 24% in the second year after treatment compared to the placebo group.
In 2012, Mondoulet et al. compared the efficacy of EPIT with SLIT in murine models sensitized to P. pratense, concluding that EPIT is as efficient as SLIT with the same allergen dose (5μg of Phl p 5) and good results in cytokine levels in bronchoalveolar lavage.276 A double-blind placebo-controlled study was published of 21 children (aged 3 months-15 years) allergic to cow's milk protein.329 During three months, three 48h applications at weekly intervals with skin patches (DBV Viaskin® Technologies) were performed. Numerous local reactions were observed although no child abandoned the study. The threshold dose of tolerance was measured and compared before and after the completion of treatment. The threshold dose was increased in the active group without statistical significance. The cumulative tolerated dose at day 0 was 1.77±2.98ml versus 23.61±28.61ml at day 90. The authors conclude that, compared to other routes for the induction of oral tolerance, this new route promises good results.
The main objective with the use of ILIT is the release of high doses of allergens directly into the lymph nodes. This route provokes powerful immune responses and has been tested in cancer therapies with animals and humans. In 2012, a phase I/IIa study with humans allergic to cat hair was published; three intralymphatic injections of rFel d 1 combined with a bacterial adjuvant (MAT-Fel d 1) were administered (monthly interval) obtaining among others a very significant improvement in clinical scores, IL-10 measures and specific IgG4. No systemic reactions were observed.330
Primary preventionAIT has shown to have a preventive effect in patients with subclinical sensitization.28 The rationale for this preventive effect has been previously described.26,27,220,223,331,332 The administration of appropriate immunotherapy from one allergen source to mono/oligo-sensitized children can reduce the likelihood of patients developing additional sensitizations from other allergen sources. This effect has been shown in retrospective and prospective studies with SCIT and SLIT.
Szépfalusi et al. recently published the first randomized, double-blinded, placebo-controlled trial that has investigated the safety, immunomodulatory and sensitization-preventive effect of SLIT in 31 mono/oligoclonally children (2–5 years of age) sensitized to house dust mite or grass pollen and clinically asymptomatic.28 The study showed that preventive SLIT is safe in these children and induces regulatory mechanisms involving allergen-specific IgG and IL-10.
The most recent study is a prospective, randomized, double-blinded, placebo-controlled trial that included 111 children with less than one year of age at risk of atopy but with negative SPT responses to common allergens at randomization.333 The main objective of the study was to demonstrate proof of concept for oral immunotherapy to high-dose house dust mite allergen in infancy in the prevention of allergen sensitization and allergic diseases. The study concluded that prophylactic SLIT is well tolerated in this population. In addition, the results met the trial's prespecified criteria of proof of concept in reducing sensitization to any allergen. However, no significant preventive effect was observed on house dust mite sensitization or allergy-related symptoms.
Ethical disclosuresConfidentiality dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Protection of human subjects and animals in researchThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Conflict of interestThe authors declare that they do not have any conflict of interest that may inappropriately influence this work.
Stallergenes Ibérica S.A. provided the financial support for medical writing services.