Assess the frequency and severity of exercise-induced bronchospasm (EIB) in obese adolescents.
MethodsA cross-sectional descriptive study involving 80 adolescents of both genders, aged 10–16 years-old, divided into four groups according to clinical history of asthma and/or allergic rhinitis and body mass index as follows: asthmatic obese (n=18); asthmatic non-obese (n=21); obese non-asthmatic (n=26); and healthy individuals (n=15). An exercise bronchoprovocation test was used for EIB diagnosis, considered positive when the forced expiratory volume in one second (FEV1) decreased ≥15% in relation to pre-exercise FEV1. Maximum percent fall in FEV1 (MF%FEV1) and area above the curve (AAC0−30) were calculated to evaluate EIB severity and recovery.
ResultsNo significant difference was found in EIB frequency between asthmatic obese (50.0%) and asthmatic non-obese (38.0%) individuals or between obese non-asthmatics (11.5%) and healthy individuals (6.7%). However, the MF%FEV1 and AAC0−30 were significantly greater in the asthmatic obese group compared to the asthmatic non-obese (37.7% and 455 vs 24.5% and 214, p<0.03).
ConclusionsObesity did not contribute to increased EIB frequency in asthmatics and non-asthmatics. However, obesity did contribute to increased EIB severity and recovery among asthmatics.
Obesity is a public health problem in the majority of developed countries,1 as well as in Brazil.2 Excess weight is considered a risk factor for cardiovascular diseases, type II diabetes and certain types of cancer.3
Currently, obesity has been associated with chronic respiratory problems, such as asthma.4 Excessive accumulation of adipose tissue in the central region, particularly around the intrathoracic wall, can alter pulmonary mechanics, leading to an increase in contractibility and bronchial smooth muscle responsiveness. Besides that, cellular adipose produces inflammatory mediators, which, at high levels, can modify the response of the respiratory vias (airways).5
The majority of studies investigating the association between obesity and asthma use respiratory symptoms for the clinical diagnosis of asthma.6,7 This procedure compromises the results, since many individuals are classified as asthmatic, even though no evidence of bronchial hyperresponsiveness (BHR) is confirmed.8 As a direct assessment of asthma, BHR is a useful measure for understanding the relationship between these two diseases, but the assessment of BHR in obese individuals demonstrates conflicting results. Urger et al.12 found a greater frequency of EIB in obese children and adolescents with no prior history of asthma. In contrast, other studies involving children and adolescents presenting positive9 or negative for asthma,10,11 identified no differences in EIB frequency between obese and non-obese individuals.
Excess weight is associated with asthma severity, suggesting that obese asthmatic individuals present greater exacerbations during asthma crises and greater use of medication compared to non-obese asthmatics.13,14 EIB severity among obese individuals, using parameters such as the MF%FEV1 and the AAC was not investigated. The purpose of the present study was to assess the frequency and severity of EIB in obese adolescents with or without prior clinical history of asthma.
Subjects and methodsA cross-sectional descriptive study composed of 80 individuals of both genders, aged 10 to 16 years-old, conveniently selected among those attending the Obesity Outpatient Service of the Endocrinology Unit and the Paediatric Allergy Outpatient Service of the Clinics Hospital, Federal University of Paraná (UFPR), Curitiba, Brazil, and public schools in close proximity to the hospital.
The participants were assigned to one of four groups, according to their body mass index (BMI) and clinical history of asthma and/or allergic rhinitis: obese asthmatics (n=18), composed of individuals presenting a BMI >85° percentile and asthma and/or allergic rhinitis; non-obese asthmatics (n=26), presenting a BMI between the 5° and 85° percentile with asthma and/or allergic rhinitis; obese non-asthmatics (n=18), presenting a BMI >85° percentile without asthma or allergic rhinitis; and healthy individuals, presenting a BMI between the 5° and 85° percentile without asthma or allergic rhinitis. The number of individuals in each group was calculated to achieve a power of 0.8, considering a difference of 5% as significant.18
The BMI was used to classify obesity, according to the scoring system proposed by Conde and Monteiro.15 Asthma diagnosis followed the recommendations of the 3rd Brazilian Consensus on Asthma Management (2002)16 and allergic rhinitis, according to the orientations of the 2nd Brazilian Consensus on Rhinitis (2006).17
BHR was assessed by the exercise bronchoprovocation test. The pulmonary function parameter measured was FEV1, before and after the exercise, at 3, 5, 10, 15 and 30min. The physical exercise was done on a treadmill (Series 2000 Treadmill, Marquette, USA), for a period of 8min, at an intensity equal to or greater than 85% of the Maximum Cardiac Frequency, obtained in a prior ergometric test. Treadmill velocity was estimated by the equation: V (mph)=1.16+0.02×stature (cm) and inclination varied between 10 and 15% (Sano et al., 1988), with readjustments made by the investigator until the individual achieved the target cardiac frequency.
Cardiac frequency was monitored by frequencimeter (Polar A1, Polar Eletro, Finland). Subjects were tested in the afternoon, between 2 and 5pm, and the environment was controlled, with temperature maintained between 20 and 25°C and air humidity less than 50%. The participants were oriented to not ingest coffee, tea and soft drinks containing caffeine for two hours prior to the test; to suspend the use of short and long-term action bronchodilators 12hours prior to testing and short and long-term action antihistamines 48hours and 5 days, respectively, prior to testing. To carry out the test, the participant should not have presented clinical symptomology compatible with viral infection (cold or flu) in the four weeks prior to testing nor be having an asthma crisis; i.e., demonstrating FEV1 values ≥80% than predicted and a FEV1/FVC ratio of ≥75%.19 The predicted FEV1 values were those determined by Polgar and Promodhat.20
For EIB diagnosis, calculation of the percentage reduction in post-exercise FEV1 values in relation to the pre-exercise values was used, as determined by the formula: %VEF1=(VEF1pre−exercise−VEF1post−exercise/VEF1pre−exercise)×100. A reduction equal to or greater than 15% was considered positive for EIB.
To assess the pattern and severity of EIB, the MF%FEV1 and the AAC0−30 were calculated. Severity was classified according to that proposed by Morton and Fitch,21 who considered a reduction in FEV1 between 10 and 24% of the pre-exercise value as mild; between 25 and 39% as moderate; and equal to or above 40% as severe.
Ethical approvalThe study protocol was approved by the Ethics Committee for Research on Humans of the Clinics Hospital of the Federal University of Paraná, under the protocol no. CEP/HC 765.184/2003-11, in accordance with resolution 196/96. All the participants were evaluated by a multidisciplinary team after acquiring the Term of Free Informed Consent from their parents or guardians.
Statistical analysesSample characterization data were expressed as the median and interquartile interval. The continuous variables were compared by the Kruskal–Wallis and Mann–Whitney tests. EIB frequency was expressed as a percentage and 95% confidence interval. The categorical variables were compared by the Fisher Exact test.22 Analysis was performed using Statistica software, version 6.0, with a level of significance set at α=5%.
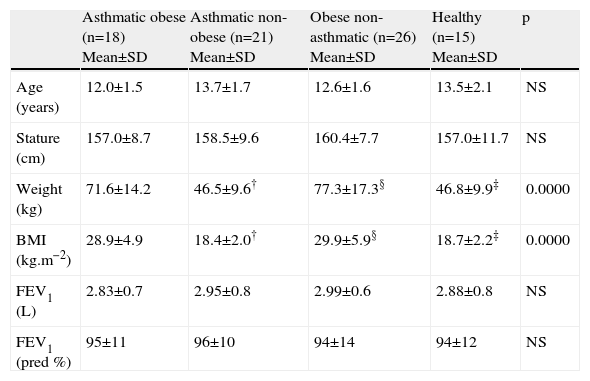
ResultsInitial characteristics of the groupsTable 1 presents the anthropometric characteristics and initial pulmonary function of the groups studied. Weight and BMI were significantly greater in the obese asthmatic and obese non-asthmatic groups, due to the presence of excess weight and obesity. Initial pulmonary function, as measured by FEV1, capacity (litres), and predicted percentage were similar among all the groups.
Anthropometric characteristics and pulmonary function of the groups
| Asthmatic obese (n=18) Mean±SD | Asthmatic non-obese (n=21) Mean±SD | Obese non-asthmatic (n=26) Mean±SD | Healthy (n=15) Mean±SD | p | |
| Age (years) | 12.0±1.5 | 13.7±1.7 | 12.6±1.6 | 13.5±2.1 | NS |
| Stature (cm) | 157.0±8.7 | 158.5±9.6 | 160.4±7.7 | 157.0±11.7 | NS |
| Weight (kg) | 71.6±14.2 | 46.5±9.6† | 77.3±17.3§ | 46.8±9.9‡ | 0.0000 |
| BMI (kg.m−2) | 28.9±4.9 | 18.4±2.0† | 29.9±5.9§ | 18.7±2.2‡ | 0.0000 |
| FEV1 (L) | 2.83±0.7 | 2.95±0.8 | 2.99±0.6 | 2.88±0.8 | NS |
| FEV1 (pred %) | 95±11 | 96±10 | 94±14 | 94±12 | NS |
BMI=body mass index; FEV1=forced expiratory volume in one second.
†Asthmatic obese×asthmatic non-obese; ‡obese non-asthmatic×healthy; § asthmatic non-obese×obese non-asthmatic.
Kuskall–Wallis and Mann–Whitney tests.
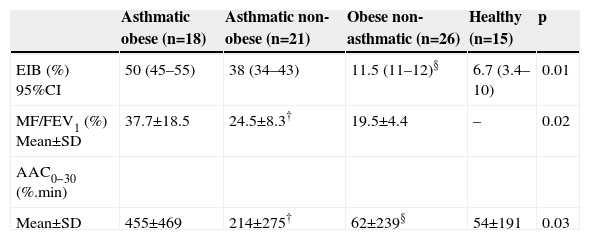
Table 2 presents the EIB frequency in all four groups. No significant difference occurred in EIB frequency between the obese asthmatic and non-obese asthmatic groups and between the obese non-asthmatics and healthy groups.
Frequency of exercise-induced bronchospasm, intensity of FEV1 reduction and area above curve in the groups studied
| Asthmatic obese (n=18) | Asthmatic non-obese (n=21) | Obese non-asthmatic (n=26) | Healthy (n=15) | p | |
| EIB (%) 95%CI | 50 (45–55) | 38 (34–43) | 11.5 (11–12)§ | 6.7 (3.4–10) | 0.01 |
| MF/FEV1 (%) Mean±SD | 37.7±18.5 | 24.5±8.3† | 19.5±4.4 | – | 0.02 |
| AAC0−30 (%.min) | |||||
| Mean±SD | 455±469 | 214±275† | 62±239§ | 54±191 | 0.03 |
EIB=exercise-induced bronchospasm; MF/FEV1=% maximum fall in FE V1; AAC0−30=area above curve.
†Asthmatic obese×asthmatic non-obese; ‡obese non-asthmatic×healthy; § asthmatic non-obese×obese non-asthmatic.
Fisher Exact test. Kuskall–Wallis and Mann–Whitney tests.
The EIB pattern and severity indicators, MF%FEV1 and AAC0−30, were significantly greater in the obese asthmatic group compared to the non-obese asthmatic group. No significant difference occurred between the obese non-asthmatics and healthy groups (Table 2).
DiscussionThe presence of excess weight is associated with numerous physiological changes that mediate the relation between obesity and asthma.
Obese individuals present systemic inflammation, with increased proinflammatory cytokines and chemokines, such as interleukine-6, leptin, interleukine-18 and tumour necrosis factor, which are involved in the etiology of non-atopic conditions like cardiovascular disease, diabetes and, potentially, asthma.4,5
Obesity is not clearly associated with allergy. However, excess weight increases the non-eosinophilic inflammatory process, which amplifies the risk of non-atopic asthma. Both FEV1 and FVC values are frequently reduced in obese adults,23 although in children and adolescents these values are similar between obese and non-obese individuals.24 In the present study, all the groups presented similar FEV1 values, above 90% of the predicted value for age and stature.
The effects of obesity can also be measured by alterations in respiratory function, given that weight increase has been prospectively associated with bronchial hyperresponsiveness in both asthmatic and non-asthmatic children.7 Studies assessing EIB in obese and non-obese children and adolescents have found conflicting results.9–12 Ulger et al.12 found a significantly greater EIB frequency among obese individuals with no clinical history of asthma compared to non-obese individuals (31.6% versus 3.3%, p=0.003). Other studies found no difference in EIB prevalence between obese and non-obese individuals.9,10
In the present work, EIB prevalence was significantly greater among obese and non-obese asthmatics in comparison with obese and non-obese non-asthmatics (50.0% and 38.0% versus 11.5% and 6.7%, p<0.05). However, when comparing the obese and non-obese asthmatic groups (50.0% versus 38.0%) and the obese and non-obese non-asthmatic groups (11.5% versus 6.7%), no significant differences were observed.
The higher EIB frequency found in the obese and non-obese asthmatic groups is related to the presence of asthma, given that EIB prevalence is higher among asthmatics than in the general population and varies between 40 and 90%.25 In this study, the presence of excess weight, both in the asthmatic and non-asthmatic groups, did not contribute to increased EIB frequencies. These results corroborate the majority of studies that observed no difference in EIB frequency between obese and non-obese individuals.9,10
In contrast, excess weight is associated with greater asthma severity, given that obese individuals present greater exacerbation of asthma crises and greater requirement for medication than non-obese individuals.13,14 EIB severity has rarely been investigated and was measured by MF%FEV1 and the AAC0−30.
Kaplan and Montana9 observed a significantly greater MF%FEV1 in non-asthmatic obese children compared to non-obese (10.4% versus 4.1%, p<0.05). Del Rio-Navarro et al.11 found a significantly greater MF%FEV1 in asthmatic obese children compared to non-obese (17.4% versus 9.0%, p<0.05). In this study, the MF%FEV1 was significantly greater in asthmatic obese adolescents in comparison with non-obese (37.7 versus 24.5, p=0.01).
EIB research on obese adolescents by the AAC presented no results regarding EIB severity. This parameter represents a prolonged EIB crisis, since it relates MF%FEV1 and EIB recovery time.26 The present study found a significantly greater AAC0−30 among asthmatic obese adolescents in comparison to non-obese (455.0 versus 214.0, p=0.01). This result demonstrates a different EIB pattern among obese and non-obese asthmatics, revealing that besides presenting a greater severity in an EIB crisis, obese asthmatics can present difficulties in the recovery of normal pulmonary function; thus limiting their participation in physical and sporting activities.
In summary, the presence of excess weight in the present study did not increase exercise-induced bronchospasm frequency among asthmatic and non-asthmatic adolescents; however, excess weight in asthmatics significantly contributed to an increase in exercise-induced bronchospasm severity and recovery period. Further studies are required to assess the effects of weight loss on exercise-induced bronchospasm severity and recovery period in obese asthmatic adolescents.
The authors would like to thank the Exercise and Sports Research Centre of the Physical Education Department, the UFPR Sports and Physical Education Centre, the Association of Friends of the Clinics Hospital and the City Government of Curitiba.