Allergic rhinitis (AR) is highly prevalent in many populations, resulting in considerable morbidity and socioeconomic costs.1 Inflammation in AR is mainly characterised by inflammatory response with eosinophil recruitment and activation in nasal mucosa.2 Although the pathophysiology of allergic diseases has been widely studied, effective primary prevention treatments are not available.
Previous studies have shown that in developing countries, where helminth infections have a high prevalence, atopy airway reactivity is negatively associated with parasite infections.3,4 In experimental models, mice exposed to different helminths have consistently shown that allergic pulmonary response to ovalbumin (OVA) was significantly inhibited.5,6 One important question raised is whether the upper airways may also be influenced by helminth exposure. This is the first study to assess the effect of helminths in a model of AR. The aim of this short report is to analyse the effect of the exposure to different parasite extracts on the influx of eosinophils in the nasal mucosa of mice with upper airway eosinophilic response to OVA.Female BALB/c mice (6–8) weeks old were used. Mice (n=45) were divided into four groups, according to the helminths they were exposed to: Angiostrongylus costaricensis (n=12); Angiostrongylus cantonensis (n=13); Ascaris lumbricoides (n=14); and one control group (n=6). Animals were housed in cages with filter-cap and in a temperature-controlled room at the animal house, with water and food ad libitum and kept on a 12-h light/dark cycle.
Adult worms were obtained from the Laboratory of Molecular Parasitology (Institute of Biomedical Research/PUCRS). The extract of worms was carefully soaked and washed separately and liquid nitrogen was then added to perform lyophilisation. Buffered solution of TRIS NaCl 20mM with protease inhibitors (TLCK, EDTA, PMSF) was used as the extraction buffer. The resulting solution was sonicated three times for 2min and then centrifuged twice (12,000rpm/1000×g) for 20min at 4°C. Supernatant was extracted and the protein concentration was estimated using the method of Bradford (Bio-Rad protein assay, USA). The concentration for intraperitoneal (i.p.) injection of protein extract was 340μg/200μL for each mouse of the groups studied. Mice were exposed to parasite extract seven days prior to OVA sensitisation.
Mice were sensitised by two i.p injections of 100μg of OVA (grade V, Sigma, USA), with 100μg of alum (aluminium hydroxide hydrate, Sigma, USA), diluted in phosphate-buffered saline (PBS, total volume: 200μL) on days 0 and 14. Ovalbumin instillation (100μg of OVA, diluted in 50μL of PBS) was micropipetted into both nostrils of each mouse, from days 25 to 27, under light anaesthesia with halothane. For histological analysis, mice were euthanised 24h after the last nasal challenge, with high ketamine/xylazine dosage injections.
In all groups, heads were separated from the body, and scalp and lower jaw were removed. Heads were fixed in 10% buffered formalin for at least 48h. After fixation, heads were decalcified in 12% ethylenediaminetetraacetic acid solution (EDTA, Sigma, USA), for two weeks. After decalcification, heads were cut through coronal sections into four regions for paraffin embedding: (A) at the incisor teeth; (B) halfway between A and C; (C) at the anterior margin of orbit; and (D) halfway between the posterior margin of the orbit and C. Tissue sections were cut at 3–4μm thickness and stained with haematoxylin–eosin (H&E).
The number of eosinophils was quantified per area (mm2) within the submucosa or mucosa of the septum or turbinates, encompassing an area of 0.0165mm2, using the software Image Pro 4.5®. The average of cells counted in six high-power fields (400×) was used for data analysis. Slides were examined by two independent observers, who were blinded to the animal groups analysed.
Data were analysed with SPSS 11.5 for Windows (SPSS Inc., USA). Data were expressed as mean±SEM. The mean eosinophil counts analysed were compared among groups using one-way analysis of variance (ANOVA) test, with Bonferroni test. Differences were considered to be statistically significant when p<0.05. This study was approved by the Animal Ethics Committee from our institution.
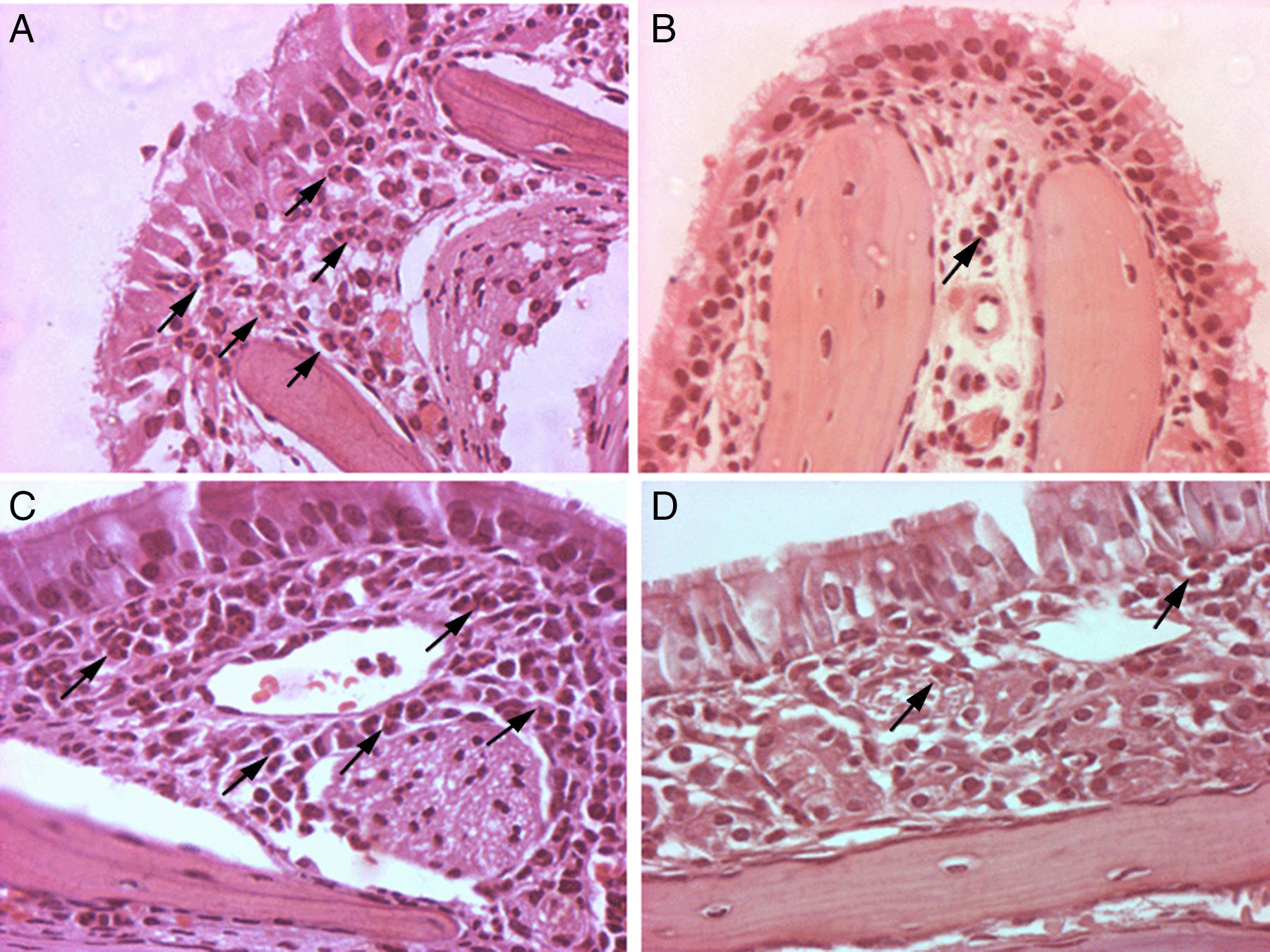
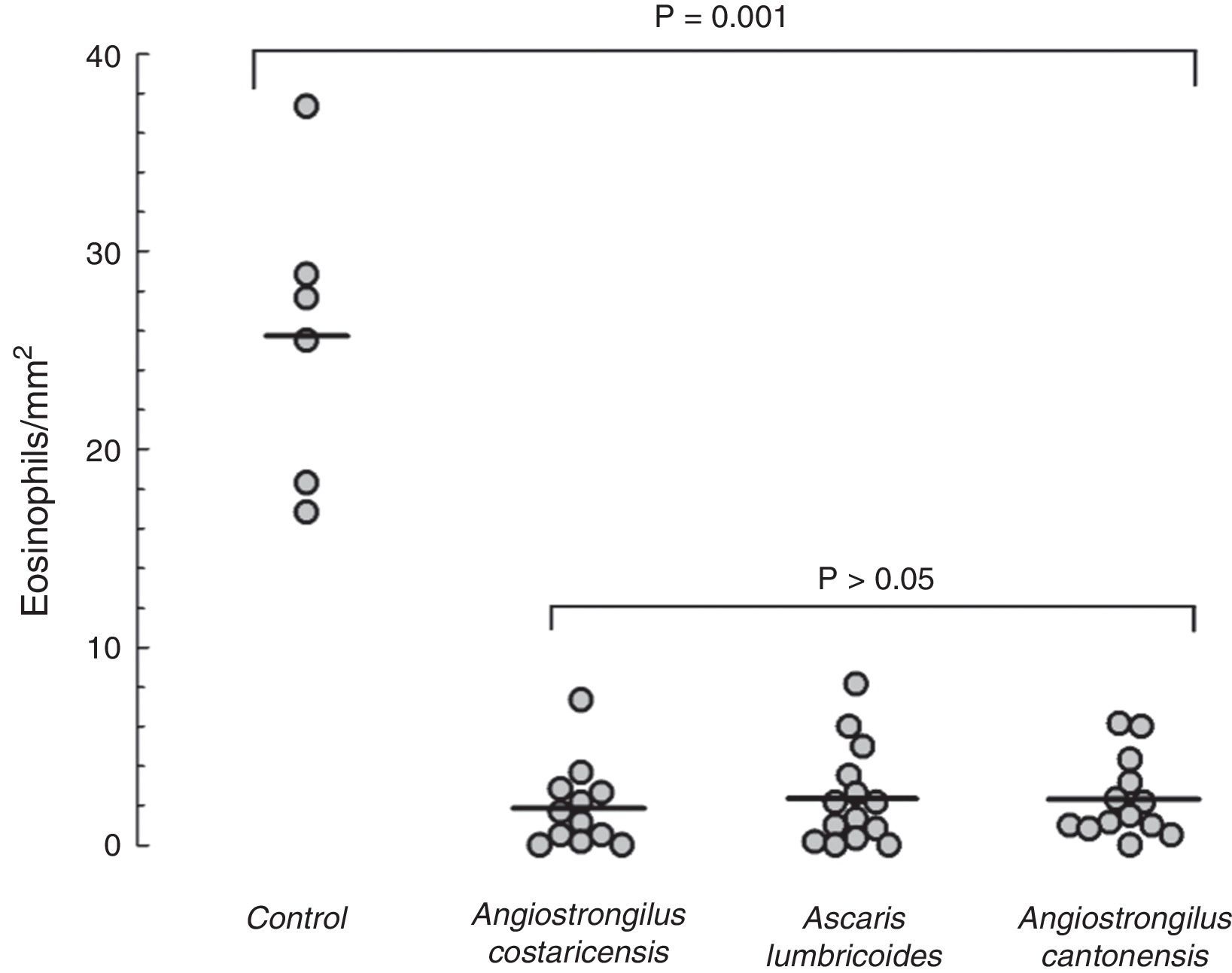
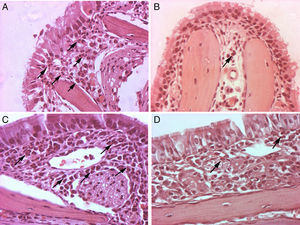
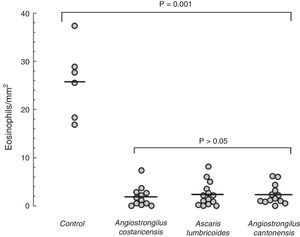
Mice from the positive control group, sensitised and challenged with OVA, developed significant upper airway eosinophil infiltration. The lamina propria was thickened, and inflammatory cells and oedema of the nasal mucosa were more intense in the positive control group. Eosinophil infiltration was almost exclusively confined to the underlying submucosa. Eosinophil infiltration was most intense in sections C and D within the lower one-half of the nasal septum and lateral nasal wall. All mice that were previously exposed to parasite extracts had very low degree of eosinophil infiltration in the submucosa (Fig. 1), and less mucosal oedema. The mean of eosinophil cell counts from the A. costaricensis group was 1.88±0.60cells/mm2. A. lumbricoides and A. cantonensis eosinophil cell counts were respectively 2.37±0.66cells/mm2 and 2.31±0.56cells/mm2. Eosinophil infiltration in parasite extract groups was significantly suppressed when compared to the control group (p=0.001; Fig. 2). Eosinophil cell counts were not significantly different among the parasite extract groups.
Photomicrographs of histopathological changes of nasal mucosa/submucosa from mice sensitised with OVA (original magnification, 400×). Twenty-four hours after OVA nasal challenge, mice were euthanised. Nasal specimens were fixed, decalcified and stained with haematoxylin and eosin. Many eosinophils were present in the nasal submucosa of the allergic rhinitis control group (A, C). Rare eosinophils were observed in the nasal submucosa of A. costaricensis, A. cantonensis and A. lumbricoides extract groups (B, D). Eosinophils are highlighted with black arrows.
Eosinophil counts in the mouse model of allergic rhinitis. Number of eosinophils in submucosal area of nasal septum and turbinates was counted under light microscopy (400×) using a software image editor. Mean eosinophil count in the control group was significantly higher than mean counts from the parasite extract groups (p=0.001). Values are expressed as mean±SEM.
We demonstrated that mice exposed systemically to distinct parasite extracts before sensitisation and challenge to OVA showed a significant suppression of eosinophilic inflammation in the upper airways, showing less non-specific inflammatory abnormalities (oedema of mucosa). These original findings offer a new approach to explain the relationship between helminths and allergic diseases.
Previous studies have focused on the interaction between the parasite infection process during the natural parasite cycle in the host and the development of atopic asthma in humans.3,4 Other reports have shown in animal models that helminths inhibit allergic pulmonary response.5–7 We have previously demonstrated that A. costaricensis infection and exposure to its extract were both associated with a decrease of airway inflammation in a murine model of allergic pulmonary response to OVA.6
The three different parasites tested in our experiment showed a similar suppression of the eosinophil influx in the upper airways mucosa. Cooper et al. postulated that the modulatory effects of geohelminth infections on allergic reactivity are markedly different for different geohelminths.8 The results of our study may suggest a “group effect” between the helminths studied, where there may be similar inhibitory effects on eosinophil airway inflammation. However, the mechanisms involved in this “anti-inflammatory response” in each group were not investigated in the present study, and we cannot rule out that different mechanisms are present in each parasite-host interaction, considering the complex interaction of the diverse scenario of parasite exposure and the development of diseases in the host.9 The most important mechanism that has been proposed to explain this inhibitory effect has been a regulatory immune response triggered in the host by helminths (i.e. IL-10/TGF-β and T-reg cell stimulation).3
Contradictory findings regarding the relationship between helminth infection and allergic have previously been discussed.8 The wide variety of populations, age of infection, intensity of infection, whether it is acute or chronic, and also the living environment are used to explain these conflicting results. However, this is the first study to assess in an experimental model, the effect of A. lumbricoides in the development of allergic response. We have shown a significant inhibitory effect in upper airway eosinophilic response in a murine model, preliminarily addressing this question more properly.
Our allergic upper airway model has been similarly reported by others. In our model, we have performed less intranasal OVA challenges when compared to previous studies. We have obtained fewer stress points prior to the endpoint compared to studies that have used a saline control group.2,10 As a result, our positive control group developed a striking localised upper airway infiltration with eosinophils, showing that this is an adequate model to study upper airway eosinophilic response to allergens and the effect of interventions.
Our main outcome is supported by eosinophil quantification per area of a sample of upper airway tissue. Eosinophils are one of the pivotal cells playing a role in the pathophysiology of allergic rhinitis. In the same line as the study by Okano et al., which used eosinophil counts as a primary outcome in a model of AR2, the authors believe that the eosinophil count analysis can be used to support our preliminary results and conclusions. It seems clear from our study that the tested helminth extracts significantly suppress eosinophil response in this allergic rhinitis model. However, more precise mechanistic analysis between helminth exposure and the development of allergy is required in further studies in order to better understand this potent inhibitory effect, such as specific IgE and T cell regulatory responses.
In conclusion, this is the first study to show that exposure of parasite extracts of adult A. costaricensis, A. cantonensis and A. lumbricoides worms strongly inhibits eosinophilic inflammatory response in a murine model of allergic rhinitis. Helminths seem to strongly inhibit the upper airway eosinophilic allergic response, and hence, our data highlight the importance of studying in more detail helminth bioproducts in order to find effective alternatives to inhibit the immune response against allergens in predisposed individuals, regardless of the heterogeneous constituent of any potential new therapy, which is a central part of the translational component of our field of research.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.