Excessive accumulation of histamine in the body leads to miscellaneous symptoms mediated by its bond to corresponding receptors (H1–H4). Increased concentration of histamine in blood can occur in healthy individuals after ingestion of foods with high contents of histamine, leading to histamine intoxication. In individuals with histamine intolerance (HIT) ingestion of food with normal contents of histamine causes histamine-mediated symptoms. HIT is a pathological process, in which the enzymatic activity of histamine-degrading enzymes is decreased or inhibited and they are insufficient to inactivate histamine from food and to prevent its passage to blood-stream. Diagnosis of HIT is difficult. Multi-faced, non-specific clinical symptoms provoked by certain kinds of foods, beverages and drugs are often attributed to different diseases, such as allergy and food intolerance, mastocytosis, psychosomatic diseases, anorexia nervosa or adverse drug reactions. Correct diagnosis of HIT followed by therapy based on histamine-free diet and supplementation of diamine oxidase can improve patient's quality of life.
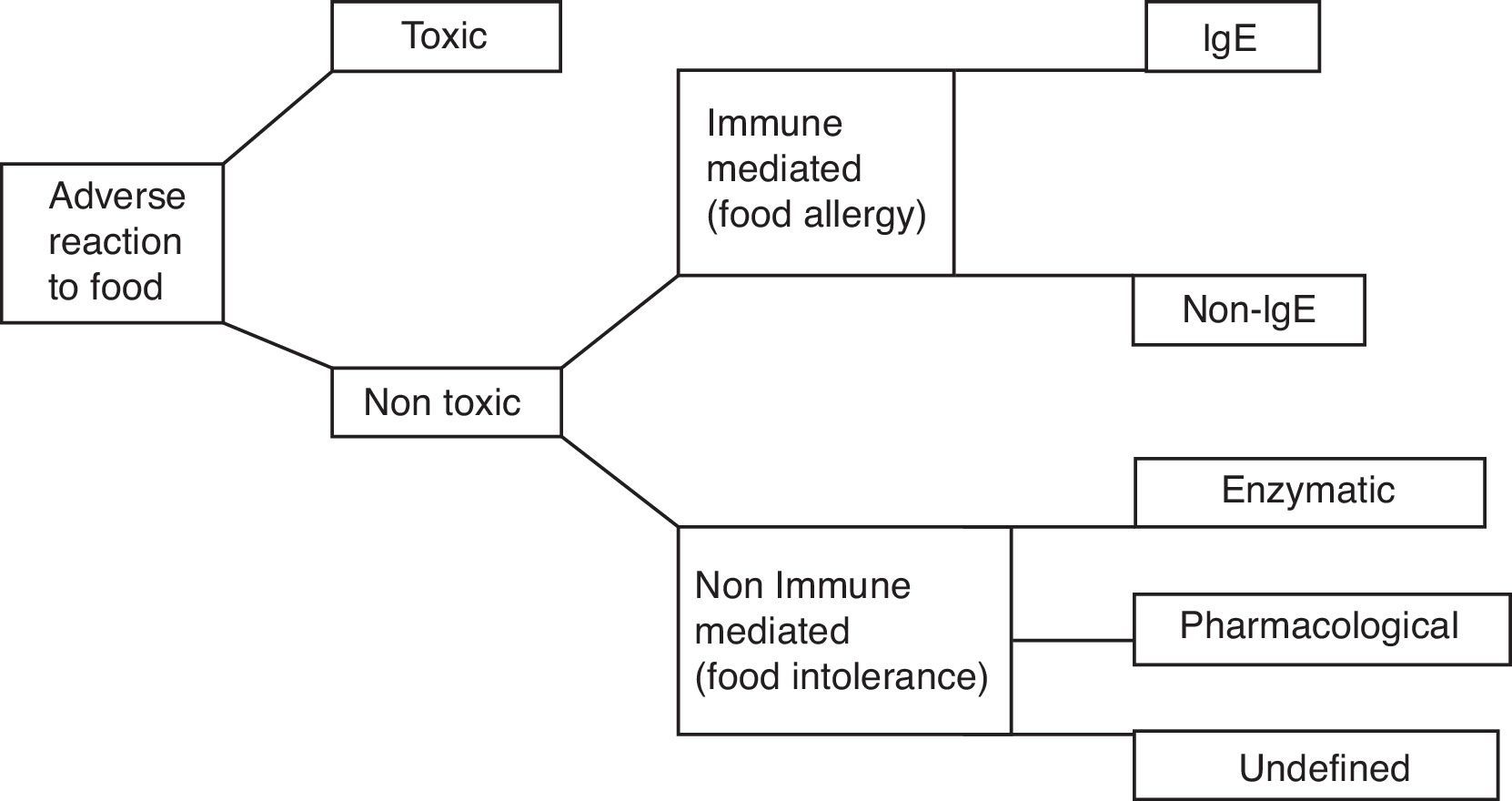
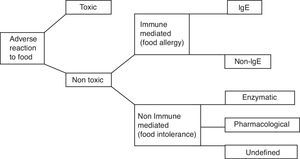
Adverse reactions of the organism to ingested food (Fig. 1) can be divided into toxic and non-toxic, caused by specific individual intolerance of food, which is generally tolerated in healthy individuals. Based on immunological mechanisms the allergic reactions occur. The most common and most severe food allergy is IgE-mediated food allergy, which occurs in predisposed individuals – atopics. Food intolerances occur in non-immune mechanisms. They can be a result of disturbance of enzymes of gastrointestinal system or as a result of pharmacologic effects of vasoactive amines present in food.1 One of these food intolerances is histamine intolerance, which is analysed in this review article.
Histamine and it's role in organismBiogenic ammine – histamine (2-[4-imidazolyl]ethylamine) has been known since 1910, when it was isolated for the first time from Claviceps purpurea by Sir Henry Dale and his colleagues from Wellcome Laboratories.2 Today, we know that histamine plays an important role in many physiological and pathological processes. Histamine causes contraction of smooth muscle cells, particularly the bronchi and intestine, dilation of vessels and their increased permeability, increases mucosal secretion, causes tachycardia and arrhythmias, influences blood pressure, stimulates secretion of gastric juices and irritates nociceptive nerve fibres. Other important processes in which histamine is involved include neurotransmission, immunomodulation (enhanced chemotaxis of eosinophils and neutrophils, production of prostaglandins and thromboxane B, suppressed synthesis of lymphokines, etc.), haemopoiesis, wound healing, intestinal ischaemia, day-night rhythm, the regulation of histamine- and polyamine-induced cell proliferation and angiogenesis in tumour models.3,4 Pleiotropic effects of histamine are mediated by its bond to membrane receptors of different cell types. Presently, there are four subtypes of histamine receptors described: histamine receptor 1 (H1R), histamine receptor 2 (H2R), histamine receptor 3 (H3R) and histamine receptor 4 (H4R). All these receptors belong to a family of receptors coupled with G-proteins. They are heptahelical transmembrane molecules, which act as transducers of extracellular signals via G-protein and intracellular system of second messengers.5
Endogenous sources of histamine in organismHistamine originates in decarboxylation of amino acid histidine mediated by enzyme l-histidine decarboxylase, which contains pyridoxal phosphate (vitamin B6).6 The name histamine comes from Greek histos – tissue, because it is present in many tissues of organism. It was isolated from liver and lung samples in 1927 by Best et al.7 Classical sources of histamine in the organism are gastric enterochromaffin cells, histaminergic neurons, mast cells and basophils, which store histamine in intracellular vesicles, from where it is released upon stimulation. It is known that degranulation of mast cells and histamine release is a result of bonding of specific antigen to Fc¿RI receptor, which can be inhibited by luteolin (flavonoid with antioxidant properties).60 Activation of mast cells can also occur in non-immune stimuli, such as neuropeptides (substance P), parts of complement system (e.g. C3a and C5a), cytokines (IL-1, IL-3, IL-8, and GM-CSF), platelet activating factor (PAF), hyperosmolarity, lipoproteins, adenosine, superoxidases and hypoxia. Many chemical and physical factors can be responsible for histamine release as well, for example extreme temperatures, trauma, vibrations or alcohol and some certain types of food and medication.8 Mast cell activation plays a crucial role in the pathogenesis of many diseases – not only allergic, but autoimmune as well, such as rheumatoid arthritis.61 Ability of de novo synthesis of histamine is also present in other cell types, e.g. platelets, monocytes/macrophages, dendritic cells, neutrophils and lymphocytes.9
Exogenous sources of histamineApart from endogenous production, histamine is introduced to the organism from exogenous sources by ingestion of some types of food, where histamine is naturally present in a high concentration. Histamine in exogenous sources can be synthesised by microbial decarboxylation of histidine by different fermenting bacteria, including natural human flora in the gut. Some bacteria are able to decarboxylate histidine in temperatures around +4°C. To prevent histamine contamination of food the cooling is insufficient, freezing and early liquidation of viable bacteria is necessary. Due to thermostability, histamine which is present in food is almost irremovable. Some types of food contain naturally high amount of histamine (cocoa, spinach, tomatoes, …). A high content of histamine is present in foods which originate by fermentation, either spontaneous or targeted (fermentation of alcoholic beverages – beer, wine; fermented vegetables, cheeses, meat, soy, yoghurt, …). It is also important not to forget bacterial contamination of food when stored improperly.10 The ability to produce histamine is present in Gram-positive, as well as Gram-negative bacteria. Many Gram-negative bacteria with this ability are common contaminants of food. From fish, ingestion of which caused histamine intoxication, the strains of Hafnia alvei, Morganella morganii, Klebsiella pneumoniae, Morganella psychrotolerans, Photobacterium phosphoreum and Photobacterium psychrotolerans were isolated. In fermented foods, the strains of Oenococcus oeni, Pediococcus parvalus, Pediococcus damnosus, Tetragenococcus species, Leuconostoc species, Lactobacillus saerimneri 30a, Lactobacillus hilgardii, Lactobacillus buchnerii and Lactobacillus curvatus are responsible for histamine production. Furthermore, it was discovered that for the contamination of ingredients in manufacturing process of wine and histamine production the strains of Lactobacillus parabuchneri, or Lactobacillus rossiae are responsible.11 Enzymatic activity of histidine decarboxylase can last even after bacterial autolysis.12
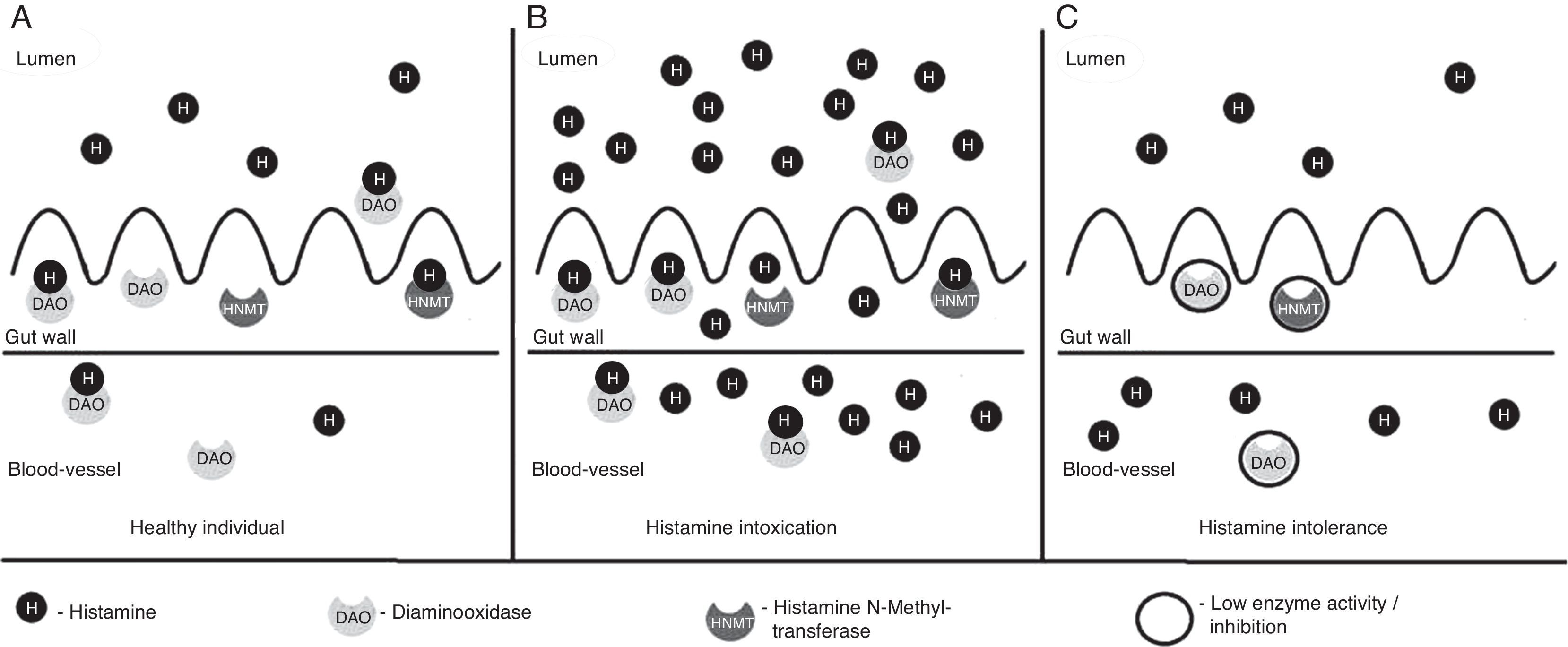
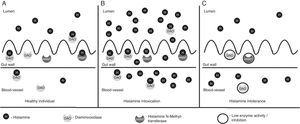
Histamine degradationBased on localisation, histamine can be inactivated by two processes – oxidative deamination of primary aminogroup to imidazolacetaldehyde, catalysed by enzyme diaminooxidase (DAO, histaminase)13 or methylation of imidazole core to N4-methylhistamine catalysed by enzyme histamine N-methyltransferase (HNMT).14 For proper function of DAO enzyme its cofactors are important – vitamins B6 and C and copper. DAO protein, stored in vesicular structures, bonds to plasma membrane of cells and is released in circulation after stimulation and is responsible for degradation of extracellular histamine. On the other hand, HNMT is present in cytosol of cells and is able to degrade histamine only in intracellular space. In mammals, DAO expression is limited to specific tissues – the highest activity of DAO is present in small intestine and ascending colon, placenta and kidneys. Decrease of DAO activity can be a potential marker of damage of intestinal mucosa by inflammatory, malignant processes or by chemotherapy. HNMT is widely expressed in many human tissues – kidneys, liver, spleen, prostate, ovaries, in cells of spinal cord, in bronchi and the trachea.8 Although both enzymes, DAO and HNMT, are present in intestinal epithelium, the main barrier of absorption of histamine into the blood stream is DAO. HNMT plays only a minor role in this process. Under normal circumstances, this enzymatic barrier sufficiently protects from resorption of histamine into blood stream (Fig. 2A).15 Diamine oxidase is continuously secreted into the intestinal lumen. Therefore, in a healthy person histamine-rich food is largely eradicated of histamine in the intestine. The remaining quantity of histamine is degraded by DAO when it passes through the intestinal mucosa. DAO also protects the body from histamine formed physiologically by intestinal bacteria in the intestine.16
Degradation of histamine in gut. (A) Healthy individual. Normal concentration of histamine in food. Most histamine is inactivated by DAO and HNMT enzymes in gut, only a small amount of histamine passes to blood stream and does not cause histamine-mediated symptoms. (B) Histamine intoxication (scombroid poisoning). Ingestion of foods with high contents of histamine (more than 500mg/kg) by healthy individual. Enzyme activity of DAO and HNMT is normal, but insufficient to inactivate excessive amount of histamine. Histamine passes to blood stream and evokes histamine-mediated symptoms. (C) Histamine intolerance. In individuals with histamine intolerance, enzyme activity of DAO and HNMT in gut is decreased or inhibited and insufficient to inactivate histamine from a food with normal concentration of histamine. Histamine passes to blood stream and evokes histamine-mediated symptoms.
Though histamine is quickly inactivated by diamine oxidase in healthy individuals, even in them severe symptoms resulting from increased concentration of histamine in blood can occur – histamine intoxication in case of ingestion of food with high contents of histamine, such as fish of fermented cheeses. Previous terms for histamine intoxication were scombroid fish poisoning, pseudoallergic fish poisoning, histamine overdose, or mahi-mahi flush. The term scombroid was used because the first fish species implicated in this poisoning were from the suborder Scombridae, which includes mackerel, tuna, marlin, swordfish, albacore, bonito, skipjack, and almost 100 other species (Scombridae is derived from the Greek word scombros, which means mackerel or tunny).17 Not only scombroid fish, but also some non-scombroid fish (mahi mahi, bluefish, sardines, pilchards…) have high contents of histamine thanks to naturally occurring histidine, which is decarboxylated by the bacteria mentioned earlier in improper processing and storage of fish. Histamine is the only biogenic amine with regulatory limits set by legislation of the European Union, up to a maximum of 200mg/kg in fresh fish and 400mg/kg in fishery products treated by enzyme maturation in brine.18 Pathogenesis of histamine intoxication cannot be explained by action of histamine alone. Scombrotoxic fish present with higher toxicity when compared to equivalent per oral dose of pure histamine. Other mechanisms are suggested, such as inhibition or potentiation of enzymes controlling histamine, presence of substances, which cause degranulation of mast cells, the presence of other histamine agonists and the existence of histamine intolerance, by which high interindividual variability in response to decomposed fish can be explained.3 Toxicity of histamine can be potentiated by other substances produced in the process of decomposition of fish – cadaverin and putrescin, which inhibit intestinal inactivation enzymes mono- and diamine oxidases19 or release histamine from bond to mucosal mucin and facilitate its absorption. Typical symptoms of histamine intoxication include rash, erythema, sweating, nausea, vomiting, diarrhoea, sensation of burning in mouth, swelling of tongue and face, headaches, respiratory distress, palpitations and hypotension. The symptoms of histamine poisoning can be present for few hours or a day, but in rare cases they may persist for several days. However, statistical data about its incidence are not available because the poisoning incidents are often underestimated due to mild or not recognised nature of illness and to inadequate systems to attribute food-borne diagnosis.18 Diagnosis is confirmed by increased plasmatic concentration of histamine in the patient or in contaminated food.20 In differential diagnosis from food allergy, the concentration of serum tryptase measured within 1–2h after onset of symptoms can be helpful.21 In food allergy, the activity of serum tryptase is increased and in histamine intoxication it is within physiological values. The course of histamine intoxication is usually mild, self-limited, lasts for short period of time and does not require therapy. In severe cases of histamine intoxication, therapeutic intervention is required – supportive volumotherapy and oxygenotherapy and oral antihistamines and bronchodilators are administered.
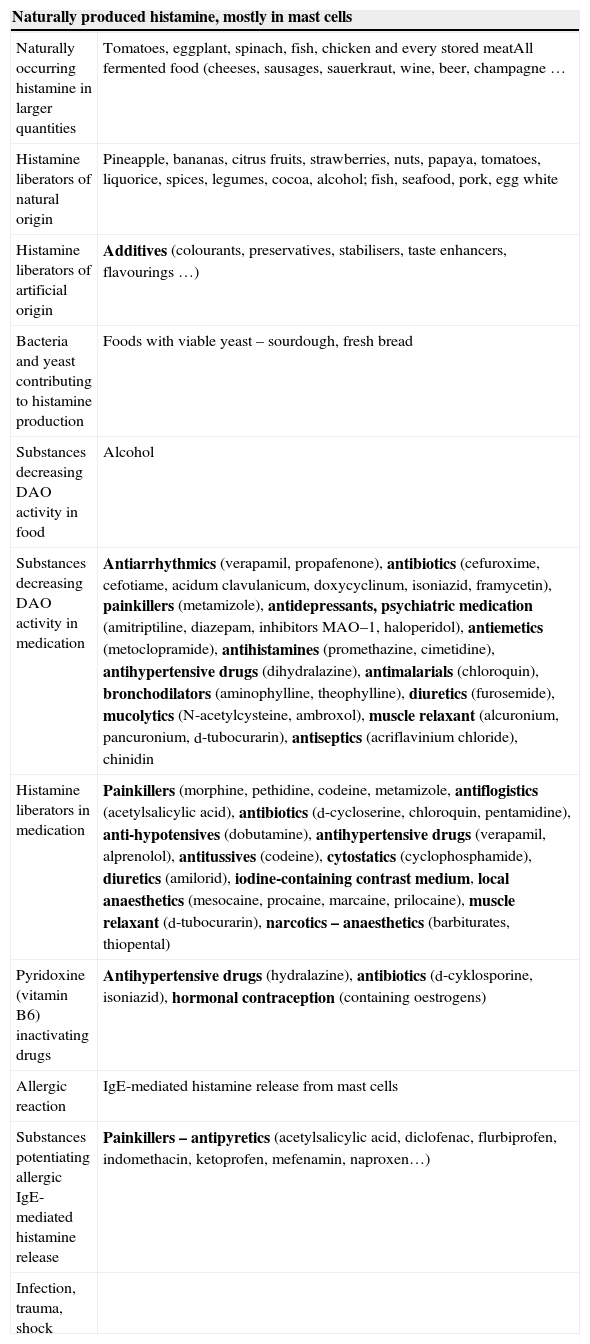
Histamine intolerance (HIT)Histamine intolerance (abbreviated HIT) is a pathological process, in which due to the disproportion between intake of histamine and ability of the organism to eliminate it, excessive accumulation of histamine occurs and development of symptoms caused by its bond to corresponding receptors (H1–H4) (Fig. 2C). HIT has typically presented more often in people who are middle-aged and prevalence of it is estimated to be 1% of the population22 although this diagnosis can be unrecognised and underestimated, because it manifests via the multi-faced clinical symptoms, which are often misinterpreted by the patient as well as by the physician. Sources of histamine and possible causes of its levels in organism are summarised in Table 1.
Sources of histamine and possible causes of increase of concentration in organism.
| Naturally produced histamine, mostly in mast cells | |
|---|---|
| Naturally occurring histamine in larger quantities | Tomatoes, eggplant, spinach, fish, chicken and every stored meatAll fermented food (cheeses, sausages, sauerkraut, wine, beer, champagne … |
| Histamine liberators of natural origin | Pineapple, bananas, citrus fruits, strawberries, nuts, papaya, tomatoes, liquorice, spices, legumes, cocoa, alcohol; fish, seafood, pork, egg white |
| Histamine liberators of artificial origin | Additives (colourants, preservatives, stabilisers, taste enhancers, flavourings …) |
| Bacteria and yeast contributing to histamine production | Foods with viable yeast – sourdough, fresh bread |
| Substances decreasing DAO activity in food | Alcohol |
| Substances decreasing DAO activity in medication | Antiarrhythmics (verapamil, propafenone), antibiotics (cefuroxime, cefotiame, acidum clavulanicum, doxycyclinum, isoniazid, framycetin), painkillers (metamizole), antidepressants, psychiatric medication (amitriptiline, diazepam, inhibitors MAO–1, haloperidol), antiemetics (metoclopramide), antihistamines (promethazine, cimetidine), antihypertensive drugs (dihydralazine), antimalarials (chloroquin), bronchodilators (aminophylline, theophylline), diuretics (furosemide), mucolytics (N-acetylcysteine, ambroxol), muscle relaxant (alcuronium, pancuronium, d-tubocurarin), antiseptics (acriflavinium chloride), chinidin |
| Histamine liberators in medication | Painkillers (morphine, pethidine, codeine, metamizole, antiflogistics (acetylsalicylic acid), antibiotics (d-cycloserine, chloroquin, pentamidine), anti-hypotensives (dobutamine), antihypertensive drugs (verapamil, alprenolol), antitussives (codeine), cytostatics (cyclophosphamide), diuretics (amilorid), iodine-containing contrast medium, local anaesthetics (mesocaine, procaine, marcaine, prilocaine), muscle relaxant (d-tubocurarin), narcotics – anaesthetics (barbiturates, thiopental) |
| Pyridoxine (vitamin B6) inactivating drugs | Antihypertensive drugs (hydralazine), antibiotics (d-cyklosporine, isoniazid), hormonal contraception (containing oestrogens) |
| Allergic reaction | IgE-mediated histamine release from mast cells |
| Substances potentiating allergic IgE-mediated histamine release | Painkillers – antipyretics (acetylsalicylic acid, diclofenac, flurbiprofen, indomethacin, ketoprofen, mefenamin, naproxen…) |
| Infection, trauma, shock | |
Under normal circumstances, there is an enzymatic barrier formed by DAO and HMNT in cells of intestinal epithelium in otherwise healthy individuals, which sufficiently protects from resorption of histamine from ingested food into the blood stream. Histamine intolerance, therefore increased histamine concentration in blood, can be caused when the amount of these protective enzymes is insufficient or these enzymes are inhibited.15 In such cases, development of symptoms resulting from increased concentration of histamine occurs even in ingestion of small amount of histamine in food, which is usually well tolerated in healthy individuals.
Insufficient activity of DAO can occur based on genetic predisposition, in diseases of gastrointestinal tract, which decrease production of DAO by damaged enterocytes (inflammatory bowel diseases, infections, parasitic infestations, dysmicrobia, metabolic malabsorption),23 or in inhibition of DAO by other biogenic amines, alcohol or medication. DAO gene polymorphisms significantly influence expression and activity of DAO, but they are not sufficient for the development of HIT on their own. Concurrence of environmental cofactors is of high importance, such as potential modifications of alternative histamine N-methyltransferase pathway, vesicular shift of both enzymes and amines or ability of enterocytes to reuptake histamine. Therefore, on the development of HIT there is a contribution of genetic, as well as environmental factors.24
Reduced DAO activity can be found in patients with chronic renal failure, viral hepatitis, advanced hepatic cirrhosis, and chronic urticaria – a typically histamine related illness with a reduced tolerance for endogenous histamine.25
Decreased degradation capacity of DAO can be caused by lack of its cofactors, vitamin B6, vitamin C, copper and zinc.26 Some substances (histamine liberators) have the ability to release histamine from endogenous reserves in the organism.10 Histamine can be synthesised from l-carnosine, which is released in the organism in physical activity and in stress in general. Dipeptide carnosine is present in tissues and is hydrolysed in stress, thus providing histamine. Studies realised in animal models suggest that in stress the concentration of carnosine decreases with a simultaneous increase of histamine concentration.27
Histamine concentration in the organism is also influenced by psychological stress. Hormones, which are released during stress reaction directly activate mast cells, which leads to the release of histamine and other inflammation factors.28,63 Apart from that, stress has negative effects on epithelium of small intestine with proved influence on activity of membrane processes and increased permeability of this important barrier. This potentiates increase of input of histamine from intestine and its liberation from mast cells by CRH-dependent mechanisms.
Because the fact that histamine is an important mediator responsible for symptoms of classical allergy reaction type I – (IgE-mediated) hypersensitive reaction, it is difficult to differentiate this reaction from histamine intolerance, which has basically the same clinical manifestation. Unlike IgE-mediated food allergy, when even small amounts of ingested antigen lead to development of symptoms, in histamine intolerance the cumulative amount of ingested histamine plays the key role.8
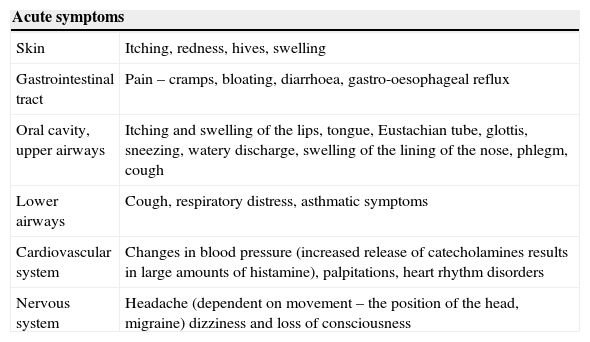
Symptoms of HITMost symptoms of HIT develop primarily due to an increase in concentration of histamine in the organism. Secondary symptoms result from the fact that increased concentration of histamine stimulates synthesis and release of catecholamins, which can cause paradoxical increase of blood pressure (even though histamine itself causes its decrease), tachycardia, dysrhythmias, nervousness, sensation of inner tremor and sleep disturbances. Signs of HIT are summarised in Table 2.29
Symptoms of histamine intolerance.
| Acute symptoms | |
|---|---|
| Skin | Itching, redness, hives, swelling |
| Gastrointestinal tract | Pain – cramps, bloating, diarrhoea, gastro-oesophageal reflux |
| Oral cavity, upper airways | Itching and swelling of the lips, tongue, Eustachian tube, glottis, sneezing, watery discharge, swelling of the lining of the nose, phlegm, cough |
| Lower airways | Cough, respiratory distress, asthmatic symptoms |
| Cardiovascular system | Changes in blood pressure (increased release of catecholamines results in large amounts of histamine), palpitations, heart rhythm disorders |
| Nervous system | Headache (dependent on movement – the position of the head, migraine) dizziness and loss of consciousness |
| Chronic symptoms |
|---|
| Chronic inappropriate fatigue |
| Dysmenorrhoea |
| Nervousness, sleep disturbances (insomnia) |
| Anxiety, panic disorder, depression |
Neurological symptoms of HIT include headaches. In patients diagnosed with migraine, increased plasmatic levels of histamine were reported not only during migraine attack, but also in asymptomatic stages of disease. In many such patients, HIT was confirmed based on decreased DAO activity. Foods rich in histamine (cheeses, wine) were triggers of headache. Limit of histamine intake in food led to disappearance of migraine symptoms.30
Nowadays we know that histamine may elicit, maintain, and aggravate headache, although the mechanisms for this are not completely understood. In some pathological processes (migraine, cluster headache, multiple sclerosis) an increased number of mast cells in brain was reported.31 Histamine does not penetrate the blood–brain barrier (BBB); however, circulating histamine may influence hypothalamic activity via the circumventricular organs that lack BBB. The study of Levy et al.32 corroborated that dural mast cell degranulation, which can be antagonised by capsaicin,62 activates a pain pathway underlying migraine headache. Most antihistamines have been shown to be ineffective as acute medication for migraine. Two centrally acting potent H1 receptor antagonists (cinnarizine and cyproheptadine) have been reported to be efficacious in preventing migraine.33 H1 receptor is vastly expressed on large intracranial arteries; it causes release of endothelial relaxation factor – NO.34 However, efficacy of antihistamines has been ascribed other actions than antihistaminergic. In addition to H1 receptors, other histamine receptor subtypes can be involved in pathophysiology of headaches. Activation of both the H3 and the H4 receptor promotes inhibitory actions on neurons. The H3 receptor causes auto-inhibition of histaminergic neurons themselves, and centrally acting H3 receptor agonist prodrugs have been shown to both inhibit neurogenic inflammation in dura, to induce sleep and to produce antinociception. Subcutaneous injections of N-alpha-methylhistamine, a catabolite of histamine with high affinity to the histamine H3 receptor, probably have some migraine preventive effect.35 A negative feedback on histamine release from mast cells in proximity to C-fibre endings has been a postulated mechanism. There are no registered ongoing studies on H3 and H4 receptor ligands in migraine.33
Brain histamine is synthesised by neurons that are restricted to the posterior basal hypothalamus, more specific to the tuberomamillary nucleus (TMN) which projects practically to the whole central nervous system. The posterior hypothalamus is the place in which several primary headaches originate. This area is initially involved in the prodromal phase of migraine attacks.36
The central histaminergic system plays an important role in the complex sleep–wake cycle, promoting cortical excitability during wakening and attention, and it consolidates the wake state. The period of the day, in the evenings and during the night, when there is reduced susceptibility for migraine attacks corresponds with less central histaminergic firing.33
Symptoms of HIT – gastrointestinal systemApart from headache, other important symptoms of histamine intolerance are diffuse pain of stomach, colic, flatulence and diarrhoea. Increased concentration of histamine and decreased DAO activity were reported not only in histamine intolerance, but also in many inflammatory and cancer diseases of gastrointestinal tract (Crohn's disease, ulcerative colitis, allergic enteropathy, food allergy, colorectal cancer).8 In intestinal mucosa of patients with food allergy, there is simultaneous decrease of HNMT. Enzymes DAO and HNMT cannot therefore compensate each other and total degradation capacity for histamine decreases.37 DAO levels are decreased also in patients with anorexia nervosa, where malnutrition causes intestinal mucosal atrophy and damage.38 On the other hand, histamine intolerance can mimic anorexia nervosa and due to similar symptoms (weight loss, diarrhoea, abdominal pain…) it can be misdiagnosed as anorexia nervosa. Accurate diagnosis and histamine-poor diet in such patients can lead to weight gain and improvement of all symptoms.39
Symptoms of HIT – respiratory tractIn patients with histamine intolerance, during or immediately after ingestion of foods with high content of histamine or alcohol symptoms such as rhinorrhoea, nasal obstruction and in extreme cases even attack of bronchial asthma, bronchoconstriction, coughing, wheezing with decrease in lung function can occur.8,40 In patients with bronchial asthma the decreased activity of HNMT was reported.41 HNMT is considered the key enzyme for histamine degradation in bronchial epithelium.42
Symptoms of HIT – skinDecreased levels of DAO in serum, symptoms of HIT (chronic headaches, dysmenorrhoea, flush, gastrointestinal discomfort, intolerance of foods rich in histamine and alcohol) were observed in patients with atopic dermatitis significantly more often when compared with the control group. Diet with limited amounts of histamine led not only to suppression of symptoms of histamine intolerance, but mitigation of symptoms of atopic dermatitis as well in these patients.26,43 Decreased activity of DAO was reported in patients with chronic urticaria as well, which is a disease mediated by histamine. Reduction of histamine in diet led to relief of urticaria symptoms.44
HIT and reproductive systemWomen with histamine intolerance often suffer from dysmenorrhoea and headaches connected with menstrual cycle. These symptoms can be explained by mutual interaction between histamine and female sex hormones and its ability to support uterine contractions. Uterus contracts at the onset of menstruation. When the effect of histamine is at its highest, such cramps in the uterus may well be triggered by histamine. This hypothesis is confirmed by clinical observation that the administration of H1 receptor blocker on the first day of menstruation may prevent pain.45 Histamine by its bond to H1 receptor dose-dependently stimulates synthesis of oestradiol and slightly influences synthesis of progesterone.46 Oestradiol has the ability to stimulate, and progesterone to inhibit production of prostaglandin PGF2α, which causes painful uterine contractions in primary dysmenorrhoea. The responsiveness of mast cells in relation to the menstrual cycle and their histamine release have also been investigated. Experiments in rats revealed high uterine histamine levels, mediated by oestradiol, and also greater uterine contractility, which might be a sign of the modulation of myometrial histamine receptors secondary to ovarian steroids.45,47 Symptom intensity of histamine intolerance can vary based on phase of menstrual cycle, with mitigation during luteal phase, when the DAO level is the highest.48
Balance between histamine and DAO is necessary for uncomplicated course of pregnancy. Due to interaction with female sex hormones, vasoactive effects, and the ability to stimulate cell growth and proliferation, histamine plays an important role in interaction between embryo and uterus during pregnancy and substantially helps by process of placental development.49 The placenta produces large quantities of DAO during pregnancy, which represents metabolic barrier preventing excessive amounts of biologically active histamine entering from the placenta into maternal and foetal circulation. Concentration of DAO in pregnant women increases by 500 times in comparison with women who are not pregnant. Due to high placental DAO production in pregnant women with histamine intolerance the symptoms transitionally mitigate during pregnancy.50 On the other hand, insufficient activity of placental DAO leads to many complications in pregnancy, such as diabetes, miscarriage and disturbances of trophoblast (mola hydatidosa, choriocarcinoma), premature rupture of foetal membranes and to premature births – these facts cause us to observe more pregnancy complications and more miscarriages in women with histamine intolerance.51
In addition, a role for histamine intolerance has been discussed in relation to sea sickness. In favour of an association is the similar risk profile (women, migraine patients), the predominantly histamine rich food at sea due to preserved foods and the therapeutic use of antihistamines.25
Diagnosis of HITDiagnosis of histamine intolerance is still quite difficult, because it manifests via the multi-faced histamine-mediated clinical symptoms (Table 3), which are often misinterpreted by the patient as well as physician and there is lack of a reliable biomarker for HIT diagnostics. These symptoms and their provocation by certain kinds of food, beverages and drugs are often attributed to different diseases, such as food allergy and other food intolerances, mastocytosis, psychosomatic diseases or adverse drug reactions. Potential food allergies should be excluded by skin prick test or by the determination of specific IgE for food allergens. Occult systemic mastocytosis should also be excluded, for example by measuring serum tryptase levels. Diagnosis of histamine intolerance requires the presentation of two or more typical symptoms of histamine intolerance.52 The diagnostic algorithm of HIT should start with a diet accompanied by careful recording of symptoms which developed after ingestion of food, identification of the exact types of food which cause the symptoms to develop and determination of histamine content in those foods. Furthermore, it is necessary to rule out other potential sources of symptom development. Improvement following the introduction of a histamine-free diet can help aid in the diagnosis of a HIT. Since exposure to histamine exists beyond diet, total avoidance of histamines is not attainable.53
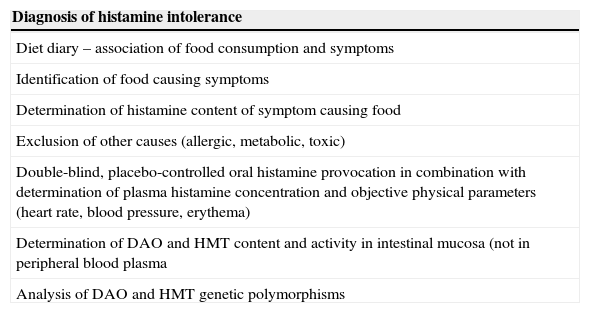
Diagnosis of HIT.
| Diagnosis of histamine intolerance |
|---|
| Diet diary – association of food consumption and symptoms |
| Identification of food causing symptoms |
| Determination of histamine content of symptom causing food |
| Exclusion of other causes (allergic, metabolic, toxic) |
| Double-blind, placebo-controlled oral histamine provocation in combination with determination of plasma histamine concentration and objective physical parameters (heart rate, blood pressure, erythema) |
| Determination of DAO and HMT content and activity in intestinal mucosa (not in peripheral blood plasma |
| Analysis of DAO and HMT genetic polymorphisms |
For determination of HIT diagnosis the use of double-blind placebo-controlled histamine food challenges with assessment of plasmatic histamine concentrations and objective assessment of symptoms developed by accumulated histamine have been proposed.22 Besides its expense in daily practice, oral provocation with histamine is very difficult to standardise. It has been published that even in patients with overt histamine intolerance, oral provocation was positive in only 50% of those tested.54 Komericki et al.55 published a multicentre study on the non-reliability of blinded oral histamine provocation to confirm histamine intolerance.
Another diagnostic method is measurement of intestinal activity of DAO and HNMT and analysis of DAO and HNMT gene polymorphisms in order to determine possible genetic predisposition.56 Determination of activity of histamine DAO and HNMT in peripheral blood is less credible, because plasmatic histamine concentration is very unstable and serum activity of DAO is decreased only in half of patients with HIT and in 17% of healthy control group.24
Kofler et al.57 propose the use of so-called Histamine-50 skin-prick-test for diagnosis of HIT, which is histamine skin prick test with readings at 50min. That study showed that patients with histamine intolerance and a control group do not remarkably differ in the size of their histamine wheals, but remarkably in their time course of the histamine wheals ≥3mm read at different time points. This difference in skin prick test allows discriminating for histamine intolerance with sufficient sensitivity and specificity.
Therapy of HITThe most effective therapy of histamine intolerance (Table 4) is limitation of foods rich in histamine. Selection of appropriate foods is often difficult, because manufacturers do not usually detail amounts of histamine in foods; it is therefore necessary to follow general suggestions. Because exposure to histamine exists beyond diet, total avoidance of histamines is not attainable.22 Furthermore, it is important to limit intake of substances, which either directly cause or stimulate endogenous histamine release and inhibit activity of DAO and HNMT. Because intolerance to medications which interfere with histamine metabolism is common, these medications should be avoided if at all possible whenever they are suspected of playing a role in causing or maintaining histamine intolerance. Where their administration cannot be avoided, e.g. in studies using contrast media, or perioperatively, antihistamines and corticosteroids should be given prophylactically.25
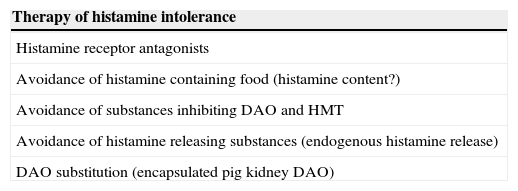
Therapy of histamine intolerance.
| Therapy of histamine intolerance |
|---|
| Histamine receptor antagonists |
| Avoidance of histamine containing food (histamine content?) |
| Avoidance of substances inhibiting DAO and HMT |
| Avoidance of histamine releasing substances (endogenous histamine release) |
| DAO substitution (encapsulated pig kidney DAO) |
Patients usually respond to a low-histamine diet in a few days, and the diet should be kept up for one month in responders (subjects no longer with symptoms); foods withdrawn are then gradually reintroduced one by one. Overall, fresh foods are advisable, whilst processed, preserved, and highly elaborated foods should be avoided. HIT is transient in many patients, who may go back to a normal diet. For more severe cases H1 antihistamines such as dex-chlorpheniramine and H2 antihistamines such as ranitidine are recommended by some authors.58 Nowadays, enzyme DAO can be per orally substituted by dietary supplements.56 Supplementation of zinc, copper, vitamin C and vitamin B6, which act as cofactors for DAO, may also be administered to improve function. In the study by Hagel et al.,59 an intravenous infusion of ascorbic acid decreased serum histamine concentrations in patients with pathologically increased histamine concentration.
In patients with most severe, practically every day manifestations of HIT, apart from diet and DAO supplementation the prevention of histamine-mediated reactions by H1 antihistamines (such as dex-chlorpheniramine) and H2 antihistamines (such as ranitidine) or cromolyn-derived medications which prevent mast cells degranulation are recommended by some authors.58 In the case of HIT, these medications are effective only in higher dosage (twice as high as used in e.g. pollinosis) and they have to be taken continuously.29
Ethical disclosuresPatients’ data protectionConfidentiality of data. The authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Protection of human subjects and animals in researchThe authors declare that no experiments were performed on humans or animals for this investigation.