Hymenoptera venom allergy is a growing problem in Spain. This problem has a special relevance in areas where population is frequently exposed to hymenoptera stings, being paediatric patients a high risk population. Immunotherapy with hymenoptera venom is an effective and safe treatment for these patients. However, there is a lack of data on the role of this treatment on paediatric population. For this reason, from the data base of the Allergy Unit from Hospital Xeral (Lugo, Spain) which includes 560 patients, have been analyzed the 21 paediatric patients, all of them treated with venom immunotherapy.
Eighteen patients completed the treatment. The maintenance dose administered was 100 μg. Two systemic reactions (both with an Apis extract) were registered. Cutaneous test and specific IgE shown a statistical significant reduction at the end of treatment (p =.0004 and p <.0001 respectively). Seven patients (33 %) suffered a spontaneous re-stung during maintenance phase or after immunotherapy was completed. In 4 patients there was no allergic reaction and the other 3 children suffered a mild local reaction.
In conclusion, venom immunotherapy is a safe and effective treatment in paediatric patients with hymenoptera venom allergy, being necessary to increase the experience on this specific segment of the allergic population.
Hymenoptera venom allergy in Spain is a growing problem: in the last 10years the number of patients diagnosed in allergy clinics has increased by nearly 100 %. Paediatric patients are at increased risk for stings1, probably because of their greater exposure. However, no studies to date have reported the exact incidence of allergic reactions to hymenoptera venom or their natural course in the paediatric population2. The problem should not be considered trivial because reports appear yearly in the lay press of deaths due to bee or hornet stings-deaths which might have been prevented if knowledge about allergic reactions to hymenoptera venom were more widespread and immunotherapy (IT) were more widely available3.
At the Allergy Unit of the Xeral-Calde Hospital in Lugo, northwestern Spain, we have compiled a database comprising a total of 560 patients with hymenoptera venom allergy, 21 of whom are children.
The decision to start IT should be based on the severity of reactions to stings and the degree of exposure4. For paediatric patients this decision may be harder to reach because traditionally children, unlike adults, have been believed to outgrow their spontaneous sensitivity to hymenoptera venom. Accordingly, a number of studies have claimed that IT is not indicated in children with mild systemic reactions involving only the skin5–7.
Published articles about hymenoptera venom allergy specifically in children are scarce2,5,8,9. The aim of the present study was thus to report our experience with an evaluation of IT with hymenoptera venom in the paediatric population at risk of being stung. Here we analyse the tolerance and efficacy of this treatment in paediatric patients diagnosed at our allergy unit as having hymenoptera venom allergy.
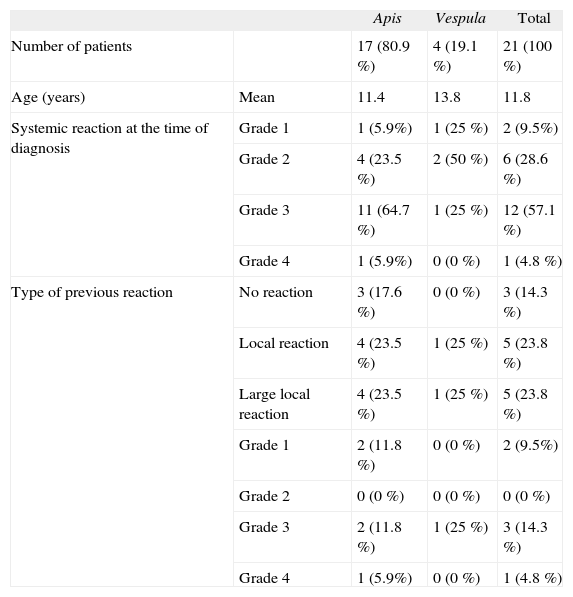
Material and methodsPatientsWe reviewed the medical records of 21 patients aged from 4 to 16years (mean age 11.8years; 8 girls, 13 boys) followed at the Allergy Unit of the Xeral Hospital in Lugo, Spain, between January 1997 and February 2008. All patients included in our data base were first seen for an allergic reaction (anaphylaxis grade 1–4 according to Müller criteria) to a bee or hornet sting. The diagnosis of allergic reaction was based on clinical history, positive intradermal skin tests and detection of specific IgE to the type of venom involved. The insect responsible for the sting and allergic reaction was a bee in 17 children (80.9 %) and a hornet in 4 (19.1 %).
ReactionsThe reaction to the sting at the time of diagnosis was a systemic reaction in all patients, and severity was classified according to Müller criteria1. The reaction was moderate to severe in 19 patients (grade 2–4) and mild in the other 2 patients (grade 1) (Table I). Table I shows the type of reaction that led the patient to seek medical care at our unit, and the type of reaction prior to the patients′ first visit at our unit.
Characteristics of patients
| Apis | Vespula | Total | ||
| Number of patients | 17 (80.9 %) | 4 (19.1 %) | 21 (100 %) | |
| Age (years) | Mean | 11.4 | 13.8 | 11.8 |
| Systemic reaction at the time of diagnosis | Grade 1 | 1 (5.9%) | 1 (25 %) | 2 (9.5%) |
| Grade 2 | 4 (23.5 %) | 2 (50 %) | 6 (28.6 %) | |
| Grade 3 | 11 (64.7 %) | 1 (25 %) | 12 (57.1 %) | |
| Grade 4 | 1 (5.9%) | 0 (0 %) | 1 (4.8 %) | |
| Type of previous reaction | No reaction | 3 (17.6 %) | 0 (0 %) | 3 (14.3 %) |
| Local reaction | 4 (23.5 %) | 1 (25 %) | 5 (23.8 %) | |
| Large local reaction | 4 (23.5 %) | 1 (25 %) | 5 (23.8 %) | |
| Grade 1 | 2 (11.8 %) | 0 (0 %) | 2 (9.5%) | |
| Grade 2 | 0 (0 %) | 0 (0 %) | 0 (0 %) | |
| Grade 3 | 2 (11.8 %) | 1 (25 %) | 3 (14.3 %) | |
| Grade 4 | 1 (5.9%) | 0 (0 %) | 1 (4.8 %) |
Skin tests were done in accordance with European guidelines with intradermal injections of a volume of 0.02ml of solution that contained 0.0, 0.01, 0.1 or 1.0mg/ml of the extract, applied on the anterior surface of the forearm along with positive and negative controls (saline solution and histamine). Tests were considered positive when the wheal, observed 20min after injection, measured at least 5mm in diameter and was accompanied by erythema.
The same allergenic extracts (Pharmalgen, ALK-Abelló S.A) were used for all skin tests and all IT injections.
In vitro testsSpecific IgE was measured with a commercial CAP assay (Pharmacia, Uppsala, Sweden) in accordance with the manufacturer's instructions.
ImmunotherapyWe used a semirush procedure for initial desensitisation, consisting of one or two weekly injections of nine increasing doses of venom (Apis mellifera or Vespula). This schedule has been previously published3. The choice of frequency of administration was based on the season of the year and risk of being stung. The maintenance dose in all patients was 100μg/ml.
Immunotherapy lasted for 5years or until allergological tests became negative. After 5years IT was stopped even if skin tests and specific IgE levels remained positive.
All patients received initial IT at our allergy unit, and after the maintenance dose was reached 6 patients were referred to their primary health care centre for further treatment after consultation with their paediatrician, and 15 patients continued to receive IT at our allergy unit.
MonitoringMost patients were seen annually for follow-up examinations in which the following information was recorded:
- 1.
Clinical manifestations: We recorded spontaneous stings by identified insects, severity of the reaction compared to severity of the reaction that motivated the first visit to our unit (i.e., before IT was started), type of local or systemic reaction according to the Müller classification1, and type of treatment of the reactions.
- 2.
Tolerance of IT: Adverse reactions to vaccination were recorded with the EAACI criteria and the Müller system1. Local reactions (immediate or delayed) were recorded as diameter of the wheal, dose that caused the reaction, treatment of the reaction, and outcome (change in the dose for subsequent injections). Systemic reactions (immediate or delayed) were recorded as dose that caused the reaction, treatment, and whether the dose was reduced because of the reaction.
- 3.
Skin tests and specific IgE: We recorded skin test results (wheal diameter in mm) and specific IgE values (in IU/ml) for each patient before and after IT. The skin test results were recorded for each concentration of extract used for each injection (0.0, 0.01, 0.1 or 1.0mg/ml).
Descriptive statistics were used to report the results for IgE measurements and skin tests before and after IT. Intragroup comparisons (before and after IT) were done with the Wilcoxon signed-rank test, and P values lower than 0.05 were considered statistically significant. Regression analysis was done with pre-treatment values for IgE and skin tests as the dependent variable in order to determine the influence of treatment on efficacy (post-treatment IgE and skin test values).
ResultsToleranceA total of five adverse reactions, three local and two systemic grade 3, were registered. All these reactions occurred using an extract of Apis. Due to this limited number of patients it has not been possible to identify possible risk factors of the appearance of adverse reactions. No adverse reactions were registered with the Vespula extract. In the 18 patients who completed IT, mean duration of treatment was 4.2years in the 14 who received one weekly injection and in the 4 who received two weekly injections. The frequency of administration was chosen on the basis of the season of the year and the risk of being stung again after IT had begun.
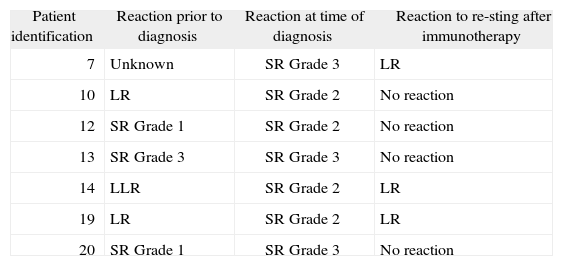
Clinical efficacyReactions in patients who were re-stung during or after the IT was completed are summarised in Table II. Seven patients (33.3 %), all of whom were allergic to bee venom, were stung again by a bee during IT: five during the maintenance phase and one a few months after completing IT. In four of these seven patients there was no allergic reaction, and in the other three children there was a mild local reaction. In all children the reaction to re-sting was weaker than the allergic reaction that motivated their diagnosis. None of the children who were stung again after IT had a reaction that was more severe than the reaction that motivated IT.
Reactions in patients who were re-stung
| Patient identification | Reaction prior to diagnosis | Reaction at time of diagnosis | Reaction to re-sting after immunotherapy |
| 7 | Unknown | SR Grade 3 | LR |
| 10 | LR | SR Grade 2 | No reaction |
| 12 | SR Grade 1 | SR Grade 2 | No reaction |
| 13 | SR Grade 3 | SR Grade 3 | No reaction |
| 14 | LLR | SR Grade 2 | LR |
| 19 | LR | SR Grade 2 | LR |
| 20 | SR Grade 1 | SR Grade 3 | No reaction |
SR: Systemic reaction; LR: Local reaction; LLR: Large local reaction.
In two patients the skin prick tests became negative after 3 and 5years of IT. When we compared skin tests before and after IT, we found a decrease in wheal diameter of 2.5mm which was statistically significant (median difference: ¿2.50, 95 % CI: ¿4.40, ¿1.33; p = 0.0004).
Specific IgEIn four patients specific IgE became negative after IT (< 0.35IU/ml). Comparing specific IgE values before and after IT we have found a decrease of 5.78 (median difference: ¿5.78, 95 % CI: ¿33.67, ¿4.81, p < 0.0001). Regression analysis performed on these data showed a statistically significant difference (r2 = 0.54, p = 0.001).
DiscussionDespite published studies on allergy to hymenoptera venom in adults, few studies have appeared which focus on this allergy in the paediatric population. Although severe allergic reactions to hymenoptera insect stings are admittedly rare7, the spontaneous desensitisation assumed to occur in children has not been consistently supported by well-designed studies. Golden et al.2 reported that in their study population the percentage of systemic reactions to insect stings in children who had not received IT was 17 %, whereas in treated patients the figure was 3 %. Valentine et al.5 found figures of 1.2 % vs. 9.2 % respectively in patients who had and had not received specific IT. In our series, 7 of 21 patients (33 %) were stung again after IT without showing any systemic reaction.
The population served by the local health service is at increased risk for exposure to hymenoptera insects. This is due, in part, to the socioeconomic characteristics of the province, where farming (e.g., vineyards and fruit orchards) and other outdoor activities such as hunting and fishing are common. In addition, a considerable percentage of the population, despite living in an urban environment during the working week, spends the weekends in rural environments where beekeeping is a traditional hobby. Often the whole family takes part in honey harvesting, an activity that involves an increased likelihood of being stung.
In the population we studied, systemic reactions at the time of diagnosis were moderate to severe in 19 patients (Müller grades 2, 3 or 4) and mild in 2 patients (Müller grade 1). When we compared patients who had been stung on a previous occasion before the sting that brought them to our allergy unit, we found that 6 had had a systemic reaction, 10 a local reaction, and 3 had not had any type of allergic reaction. Two patients were unable to recall having been stung before. These findings confirm the difficulty of predicting individual responses in different patients, and together with the fact that one third of the patients in our series were stung again after IT, emphasize that the decision as to whether IT is indicated for children who have had only mild systemic reactions should be made on a case-by-case basis. The decision should take into account the patient's personal circumstances and the type of environment (e.g., urban vs. rural) where the patient usually lives.
The efficacy of IT with insect venom in our paediatric patients is supported by the finding that 33 % of our patients who were stung again after IT had no systemic reactions, and also by the overall decrease in their skin prick and specific IgE reactions. In four patients specific IgE titer became negative, and in two the skin prick tests became negative.
Our allergy unit has 15years' experience administering a semirush procedure with an aqueous hymenoptera venom extract. When we analysed tolerance in our paediatric patients, we found that two children (9 %) had a systemic reaction during the initial phase of IT. One child required adrenaline and the other was treated with antihistamines and bronchodilators. In both children we resumed IT with the previously tolerated dose, and therapy was adequately tolerated thereafter. Two children (9 %) had large local reactions which were treated with ice packs, and neither child required a change in the immunisation schedule.
After the initial phase of IT, six of our patients were referred for maintenance therapy with specific vaccines to their primary health care centre. Immunotherapy was continued in close collaboration with the paediatrician, and no adverse reactions were recorded in any of these children.
There may be reluctance to indicate IT for children because of the prospect of initiating costly, lengthy treatment for a diagnosis with a presumably favourable prognosis. Some authors10 have suggested that sequential provocation with live insect stings should be the main parameter for deciding whether IT is indicated in children. Apart from the ethical issues which provocation testing may involve, we do not support this approach, for several reasons. The amount of venom injected during a sting depends not only on the duration of the sting, but also on the age of the insect and amount of venom in the venom sac at the time of the attack11,12. Beehives are usually the source of insects used at allergy units for provocation testing. Beekeepers are aware that bees which remain in the hive are younger; consequently their venom sac contains less venom than older bees13. The amount of venom can be significantly reduced if the insect has stung previously without losing its sting. When threatened (as may occur during provocation testing), hornets can eject part of their venom without stinging. A negative result in provocation testing may thus not be solid enough evidence to rule out a systemic reaction after a subsequent sting14. From an immunological point of view, repeated provocation may itself induce sensitisation or have a booster effect in patients who later receive IT. One third of our patients were stung after they had completed IT, an incidence which suggests that the risk of being stung in our setting is high, and that, we feel, makes daily provocation testing in the laboratory unnecessary.
Few studies have investigated the efficacy and safety of IT with insect venom in children, probably because of the assumption that children outgrow their allergy, despite evidence to the contrary. The present study provides data on the safety and efficacy of a semirush procedure for patients who had severe systemic reactions. The cost-benefit ratio appears reasonable in the light of earlier publications and available data. In children who experience a mild systemic reaction, the indication for IT should be decided on a case-by-case basis with due regard for the child's living environment, parents′ profession and risk of exposure.
We thank K. Shashok for translating the original manuscript into English. Ms Shashok's fee was paid by ALK-Abello, Madrid.