INTRODUCTION
Metronidazole is a 5-Nitroimidazol compound introduced in 1959 to treat Trichomonas vaginalis infections. It is also used in application to amebiasis and anaerobic infections combined with other antibiotics such as aminoglycosides. It is generally well tolerated despite its wide usage. The most frequent side effects are gastrointestinal disorders (nausea, vomiting, diarrhea and, exceptionally, reversible pancreatitis). Reversible haematological alterations have also been described along with disorders of the central nervous system. More exceptionally there can be cutaneous hypersensitivity reactions for all fixed exanthemas. We present four cases of cutaneous exanthemas due to metronidazole.
MATERIAL AND METHODS
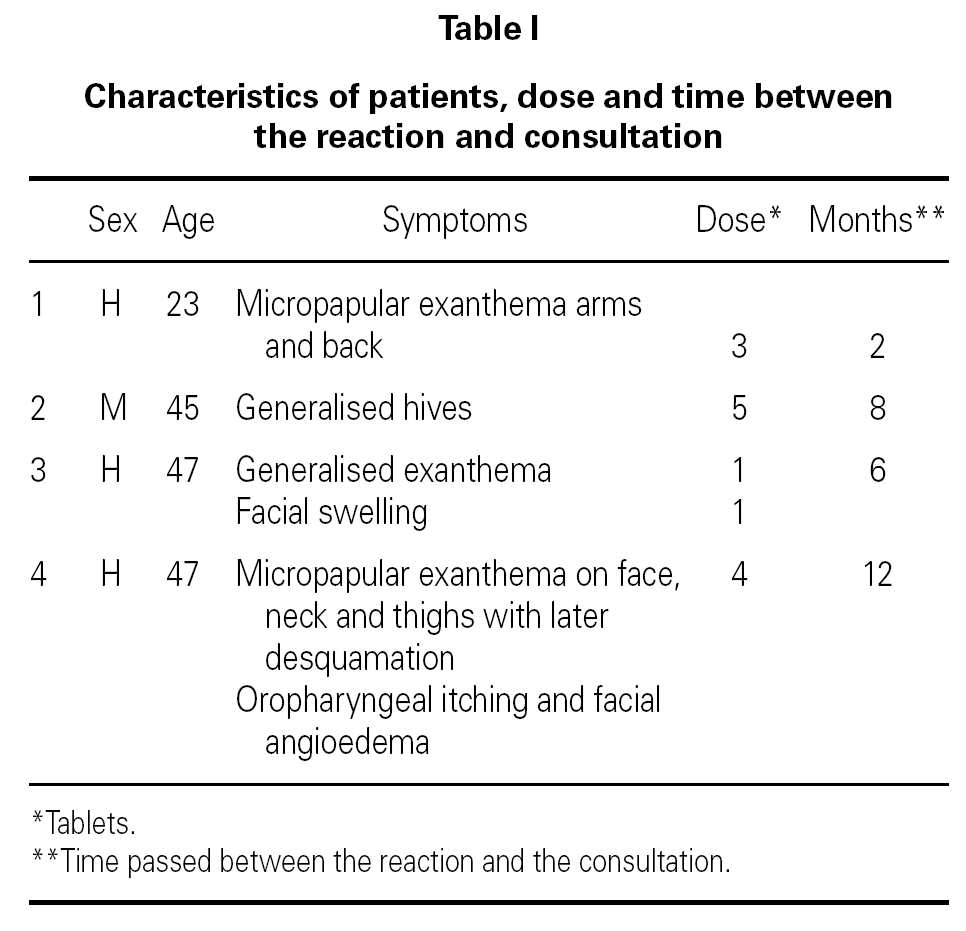
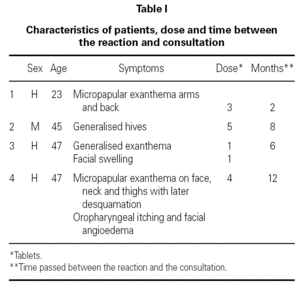
We present four patients with an average age of forty who developed cutaneous itching exanthemas (two early and two delayed) after taking Rhodogil® (125 mg metronidazole and 750.000 IU spiramycin) for mouth infections. Two of them also had facial angioedema. The symptoms disappeared in all cases with the administration of corticoids and/or antihistamines. None of these patients had consumed alcohol beforehand.
The average time elapsed between the reaction and consultation was seven months with the average accumulated dose of two tablets (table I).
Cutaneous prick tests were carried out with spiramycin and metronidazole at a concentration of 250 mg/ml and 125 mg/ml respectively together with epicutaneous tests with metronidazole at 0.5 %, 5 % and 10 % (readings at 48 and 96 hours). For both tests the ten negative controls were patients with supposed adverse drug reaction, symptoms which were later not confirmed by oral provocation (OP). Simple blind oral provocations were then carried out with placebo according to the stablished Department protocol with erythromycin, spiramycin and metronidazole at up to therapeutic doses. The last patient was not given OP with metronidazole due a positive prick test.
RESULTS
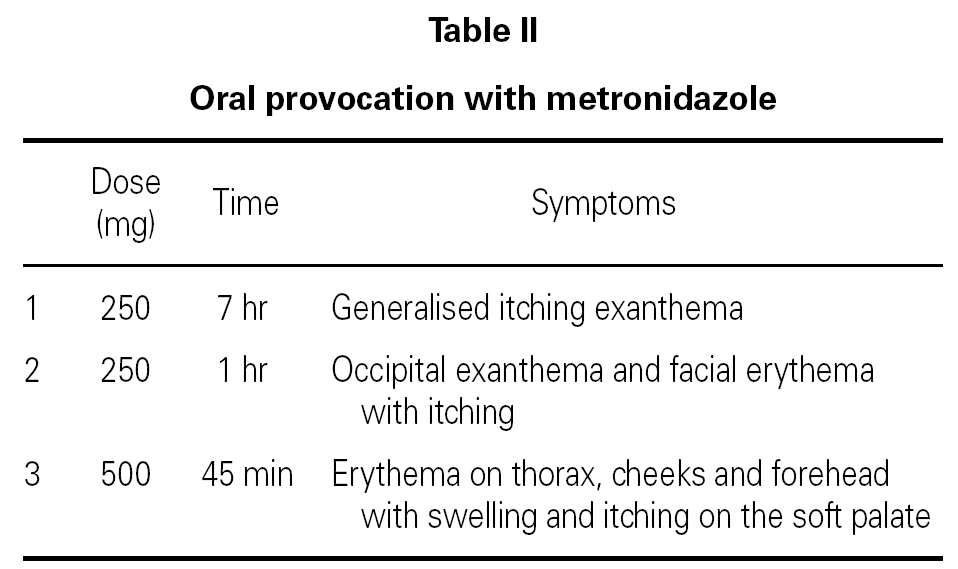
The prick test for spiramycin and the epicutaneous tests with metronidazole were negative. The prick test with metronidazole was only positive in the last case. All patients tolerated both erythromycin and spiramycine. Oral provocation with metronidazole proved positive in the first patient who developed delayed exanthema while the other two showed erythema and early itching (table II).
Oral provocation with metronidazole proved positive with a total accumulated dose of 250 mg in the first two cases and 500 mg in the third. The first patient presented generalized exanthema after seven hours, with intense itching that improved after twenty-four hours with antihistamines. The second developed facial itching an hour later, in the occipital zone and on the arms, becoming facial erythema and exanthema in the occipital zone. These manifestations disappeared after administering corticoids and antihistamines. The third patient, forty-five minutes later, developed erythema on the chest, cheeks and forehead with intense itching of the palate. These manifestations subsided within one hour after administration of intravenous corticoids and antihistamines. At examination, we observed a discreet swelling of the soft palate.
DISCUSSION
We have presented four cases of cutaneous exanthemas due to metronidazole (two early and two delayed), probably mediated by an immune mechanism which we have only been able to demonstrate in one case.
There are very few published cases of immediate hypersensitivity to metronidazole 1-4. Rash and itching type reactions have been described, particularly with topical metronidazole for the treatment of rosaceous acne, one case of exanthema similar to pityriasis rosea and two of acute generalized exanthematic pustulosis 5,6. In one of them the epicutaneous test with metronidazole at 0.75 % proved positive after seventy-two hours. Some cases have also been published involving serum disease and toxic epidermal necrolysis/Steven-Jhonson Syndrome among philippine workers in Taiwan who received high doses of metronidazole combined with mebendazole 7. However, the majority are cases of fixed exanthemas linked to medication. In some cases positive epicutaneous tests have been recorded with metronidazole at different concentrations 8,14,15.
Initially disulfiram-type reactions had been described (Antabus effect) when alcohol had been consumed concomitantly. It is thought that this is due to blocking of the hepatic dehydrogenase aldehyde enzyme and to later accumulation of acetaldehyde in the blood. Two publications have recently questioned this type of reaction but their authors believe it necessary to conduct new studies to corroborate these results 9,10. None of our patients had ingested alcohol.
On many occasions it is not easy to determine from the clinical history whether the reaction is mediated by IgE or is a delayed reaction, since the patients do not remember exactly how much time has passed between taking the medication and manifestation of the reaction when the latter is only cutaneous. Furthermore, at the time of consultation, the cutaneous symptoms have disappeared and the patients are not always able to provide the report from at the first medical visit. Thus, it is necessary to carry out cutaneous tests: prick and/or intradermal and epicutaneous tests.
Cutaneous prick tests have been described with different positive antibiotics, though on many occasions there is a lack of information on the methodology used or the clinical symptoms involved 11. Despite their great use in confirming the etiopathogenic mechanism of type I reactions, the sensitivity of these tests is not particularly high. We have found no other cases in the literature of positive prick tests for metronidazole.
Regarding the epicutaneous tests with antibiotics, their performance is not as high as in the cases of contact dermatitis 12. Positive results have been described for ECT particularly with certain drugs such as betalactams, antiepileptics drugs, benzodiazepines and nonsteroidal antiinflammatory drugs 13 but there is a lack of information on other medications. The majority of the positive epicutaneous tests with metronidazole were obtained from a fixed exanthema residual lesion 8,14,15.
Two cases have been described of fixed exanthema from tinidazole with positive oral provocation for metronidazole 16,17, though other authors have failed to confirm this 18. On also taking into account the high structural similarity between metronidazole and its derivatives (tinidazole, secnidazole and ornidazole), the possibility of cross-reactivity is seen to be very high. For this reason, and because other therapeutic alternatives are available, the possibility of provoking our patients with these derivatives is not considered.
Taking into account the low sensitivity of cutaneous testing (both prick tests and epicutaneous tests), oral provocation appears to be an essential tool for the diagnosis of early and delayed hypersensitivity reactions to metronidazole. We believe that in our cases, after oral provocation the symptoms did not reappear in the same way as in the initial reaction possibly because joint administration with spiramycin modifies haptenization of the drug after its hepatic metabolization.