Cow's milk allergy (CMA) occurs mainly during the first years of life and is one of the most prevalent food allergies in many countries. In our country it is by far the most common food allergy in children. An accurate diagnosis is necessary in order to protect children from either inadequate or unnecessary avoidance diets. This usually requires the performance of oral food challenges, exposing the patient to the risk of a severe allergic reaction. Moreover, food challenges are time consuming procedures, especially when they are performed in double blind controlled protocols 1,2.
Methods for determination of serum allergen specific IgE (sIgE) have been improved over the past years and the possibility of quantitative determination of food specific IgE opened new perspectives on the diagnosis and follow-up of these patients. Specific IgE decision points with a high predictive level of clinical reactivity to foods like milk, egg, fish and peanut have been established 3-5. However, the decision points obtained for the same allergens are considerably different, presumably depending on the characteristics of the population studied, such as age and type of clinical manifestations 6. The method used by these groups was the same UniCAP®(Pharmacia, Uppsala, Sweden).
A new method for sIgE, IMMULITE®2000 3gAllergy (Diagnostic Products Corporation, Los Angeles, USA) has been introduced recently 7,8. In this study, we evaluated the clinical performance of IMMULITE 2000 with respect to detection of cow's milk allergy by comparing it with UniCAP and by verifying, within our population, the behavior of both methods, at two different decision points (cutoffs) as previously proposed by other authors 3,4.
METHODS
Patients
Among the children on follow-up at our outpatient unit for CMA we selected those with indication to be submitted to a follow-up challenge, according to the clinical criteria in use at our department (table I). We included 31 patients (17 males, 14 females) aged between 1 and 9 years old (median: 3,5 years) - group A. All cases presented with immediate type reactions--urticaria, angioedema, vomiting or bronchospasm--and all had positive reactions to skin prick tests for whole milk and/or protein fractions (from IPI-ASAC Spain: milk, casein, alpha-lactoalbumin and beta-lactoglobulin). The diagnosis in each had been previously confirmed by positive food challenges.
For the control group (group B), we selected children with atopic dermatitis because this is a situation where it is common to find low levels of food specific IgE, even in patients without food allergy. In this group we included 19 children (10 males, 9 females) aged between 2 and 14 years old (median: 5,0 years), without clinical evidence of CMA, followed by us for other allergic diseases (atopic dermatitis in 19, asthma in 9, rhinitis in 9). The atopic dermatitis was mild and intermitent in all cases, with symptom free periods despite a daily regular intake of dairy products.
Cow's milk oral challenges
During the study all patients underwent reevaluation, which included an oral milk-challenge test. Informed consent was obtained from the parents, according to the recomendations of the hospital ethics committee. Given the young age of the children and because we expected immediate and objective reactions, we decided to use an open protocol. All challenges were performed under medical surveillance at the day-hospital unit, with appropriate medication and resuscitation equipment available. Increasing doses of cow's milk infant formula (1, 5, 10, 20, 40, 80 ml with a repeat dose of 80 ml, to a cumulative dose of 236 ml) were given at 30-minute intervals. The challenge was discontinued and considered to be positive if a clinical reaction appeared (urticaria, angioedema, erythematous rash, vomiting, diarrhea, bronchospasm, rhinoconjunctivitis or hypotension). In instances of a clinical reaction, treatment was provided according to the type and severity of symptoms. After the challenge, the child remained under observation for at least six hours. If the challenge was negative, dairy products were freely introduced to the child's diet and a new visit was scheduled within a week to check for the development of late symptoms.
Children from the control group were not challenged since they were regularly consuming dairy products without evidence of an adverse impact on their allergic disease.
In vitro tests
A blood sample was obtained from each child close to the challenge test--within approximately one month. Each blood sample was tested for cow's milk specific IgE using the IMMULITE 2000® and the UniCAP®.
IMMULITE 2000 is a third generation sIgE two-step chemiluminescent enzyme immunoassay which uses a solid phase (bead). Both the IMMULITE 2000 and UniCAP assays were performed according to the manufacturer's instructions. The results were considered positive when a level of, at least, 0.35 kU/L was obtained.
RESULTS
During the study, all children in patient group A were rechallenged to assess their state of tolerance. Of 31 milk challenges, 22 were positive (current CMA) and 9 were negative (past CMA). Samples for sIgE analysis were taken from all 50 children, (current CMA, past CMA and controls).
The values obtained for milk specific IgE in patient group A ranged from the detection limit of 0.35 kU/L, to the upper limit of 100 kU/L with UniCAP® and from below the IMMULITE® 2000 detection limit of 0.15 to the upper limit of 100 kU/L. Two patients had negative results (< 0,35 kU/L) with IMMULITE 2000 and three with UniCAP. In the control group B, the values were under 1.1 kU/L for IMMULITE 2000 and under 1.6 kU/L for UniCAP. Four patients had low positive results with both methods.
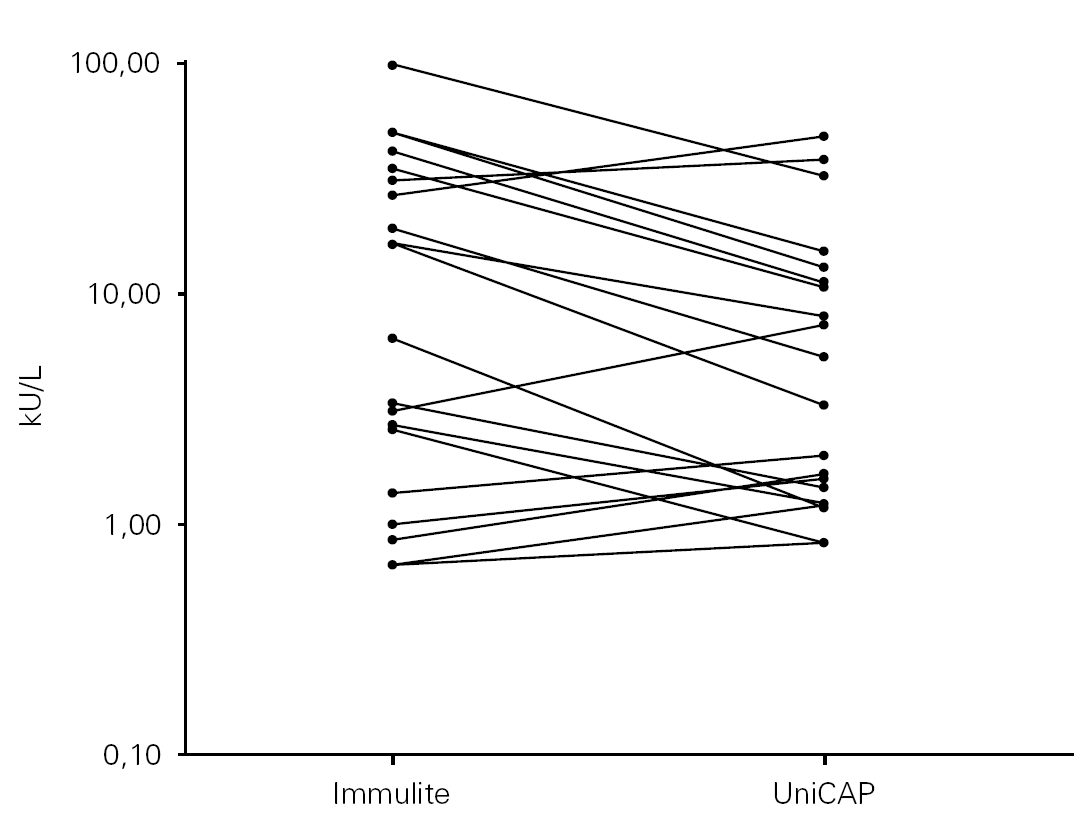
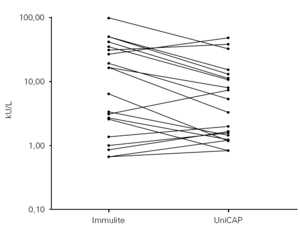
Any value at or below the detection limit of 0.35 kU/L, or at or above the upper limit of 100 kU/L for either assay, was considered to be out of range. Using these criteria, just 20 of the 50 paired samples fell within the common 0.35 to100 kU/L range by both in vitro assays. Working in a logarithmic scale, we compared the results for the 20 in-range samples, noting a trend towards reporting higher concentrations of sIgE by the IMMULITE 2000 assay in most patients (fig. 1).
Figure 1.--Cow's milk specific IgE comparison between methods for the 20 in-range samples (results within 0.35 and 100 kU/L).
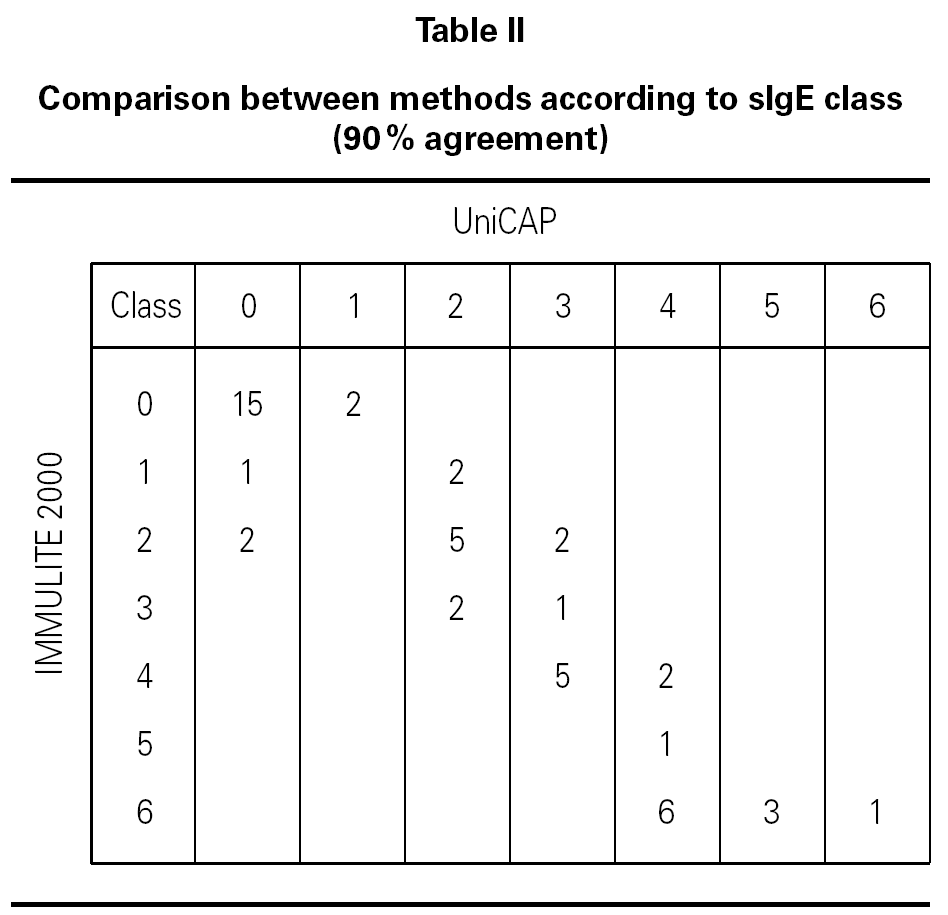
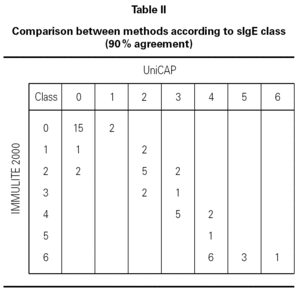
We evaluated the agreement between methods in terms of sIgE class (table II). In this case, as the analysis is qualitative, not quantitative, we used data from all 50 children. Considering a class 0 result as negative and classes 1 through 6 as positive, we obtained 90 % agreement with only five discordant results. One of these five belonged to group A and was positive (class 1) according to the IMMULITE 2000 assay, but negative using UniCAP. The challenge test for this patient was negative, indicating that he had achieved clinical remission. The additional four discordant cases were from group B. Two were falsely positive according to UniCAP (class 1) and the other two, using the IMMULITE 2000 (class 2).
We observed good agreement between methods in the lower classes, with results concurring either within the same class or within one class difference. We observed higher results with IMMULITE 2000 in the upper classes; specifically six patients designated as class 6 according to IMMULITE 2000 were designated as class 4 using UniCAP (all were positive by challenge test).
To calculate the diagnostic performance of the methods, we included only the 22 patients with active disease (positive challenge) and the 19 controls. Samples were considered positive if values were above 0.35 kU/L by either method and were compared with the results of the food challenge test. In this sample of 41 patients (CMA prevalence = 53.6 %), both methods demonstrated the same performance: 100 % sensitivity; 78.9 % specificity; 100 % negative predictive value; 84.6 % positive predictive value; 90.2 % efficiency.
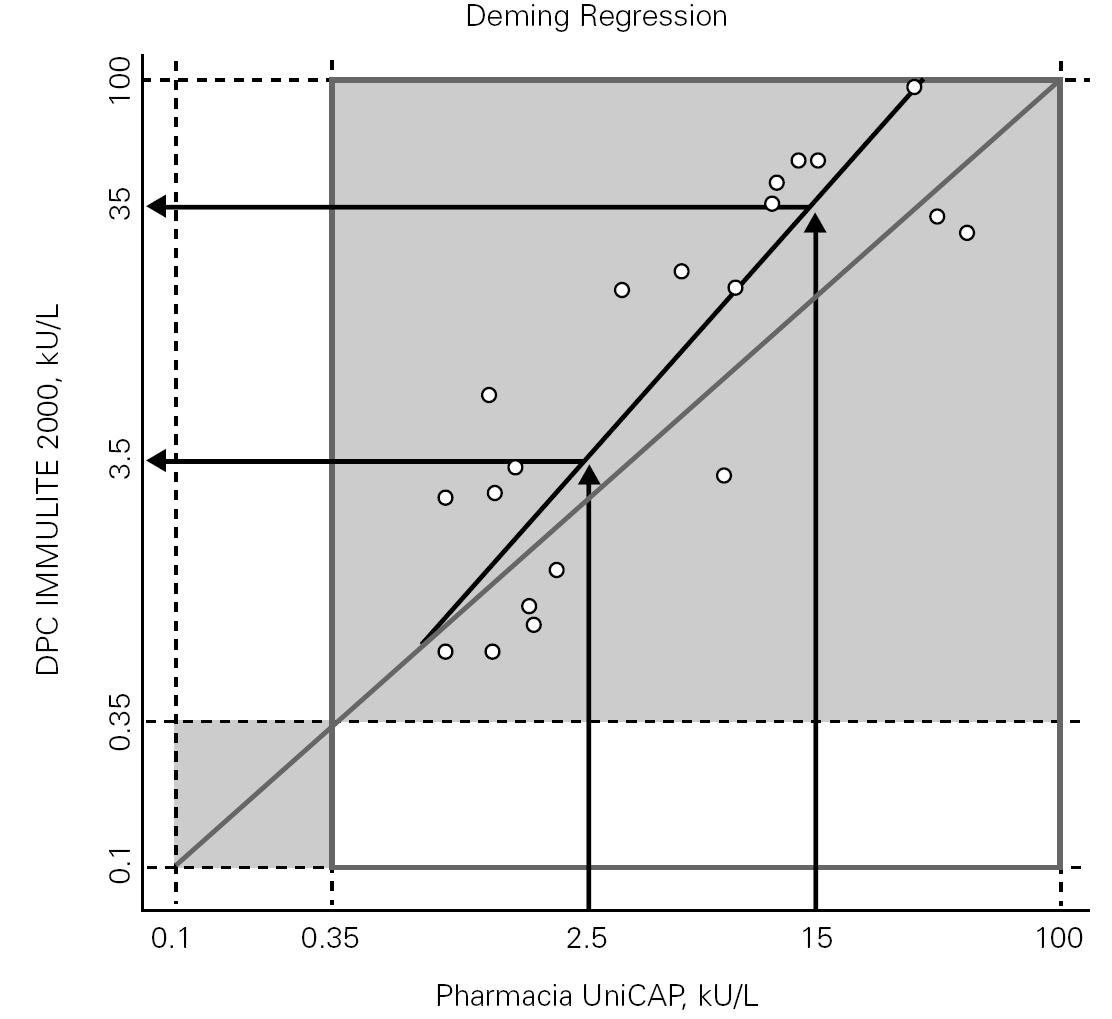
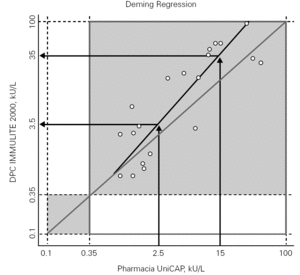
We also evaluated the behavior of both methods at two different decision points previously proposed in the literature (Sampson et al3, Garcia-Ara et al4). The results also showed a distribution with a fairly clear trend encompassing both of the cutoffs proposed for the Pharmacia system. We applied various symmetric (invertible) regression analyses to the data, using them to predict equivalent cutoffs for the DPC system. Corresponding to the proposed levels of 2.5 and 15 kU/L for UniCAP, the regression analyses predicted the following levels for IMMULITE 2000: 3.5 and 25 kU/L by Deming (fig. 2); 3.6 and 33 kU/L by OLS Bisector (not shown); 5.6 and 44 kU/L by Passing-Bablok (not shown).
Figure 2.--Prediction of cutoffs for the IMMULITE® 2000 3gAllergy corresponding to published cutoffs for UniCAP® as determined by Deming symmetric regression analyse.
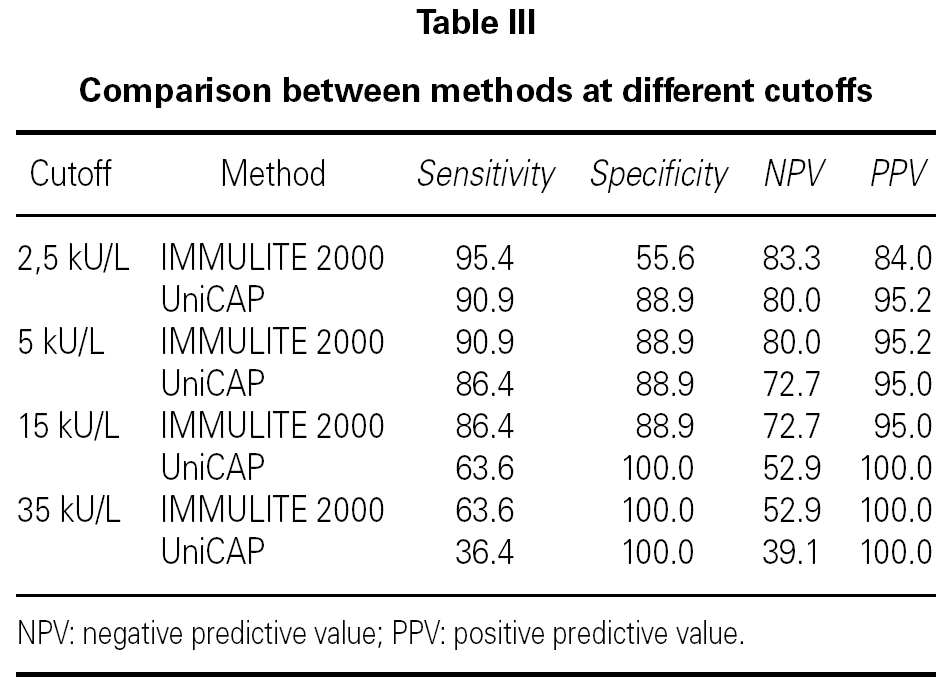
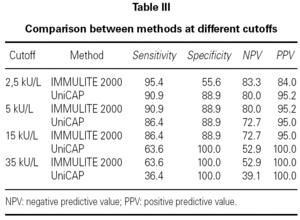
In order to verify how well the two methods discriminated between children with current and past CMA, we included only those patients in group A within our analysis (table III). At the cutoff decision point of 15 kU/L (as proposed by Sampson 3 for milk reactivity) we observed that, in our population, UniCAP shows a slightly better positive predictive value (100 %) than IMMULITE 2000 (95 %), although UniCAP's sensitivity was much lower than IMMULITE's at this cutoff (64 % vs. 86 %, respectively). However, we verified that we could obtain exactly the same positive predictive value (100 %) with IMMULITE 2000 by choosing a higher cutoff point of 35 kU/L, albeit at the cost of sensitivity (64 %). Similarly, at the much lower cutoff of 2.5 kU/L proposed by Garcia-Ara 4, UniCAP shows a somewhat better positive predictive value than IMMULITE 2000 (95.2 % vs. 84.0 %) but once again with lower sensitivity (90.9 % UniCAP vs. 95.4 % IMMULITE 2000). However, table II demonstrates that the performance and sensitivity of IMMULITE 2000 exactly equals UniCAP's performance if we increase the cutoff to 5 kU/L.
DISCUSSION
In our study population, IMMULITE® *2000 demonstrated reliable results in the detection of cow's milk specific IgE and its performance was found to be comparable to that of UniCAP®. IMMULITE 2000 demonstrated a tendency to report results for sIgE at greater concentrations, which was more evident in the higher range of results. However, at the usual positive cutoff of 0.35 kU/L, both methods detected all cases of CMA (100 % sensitivity). The specificity was also equal for the two methods and, although somewhat lower (78.9 %), it was acceptable, considering the particular characteristics of the control group. For this group, we deliberately chose children with atopic dermatitis because this is a clinical situation where we often find high levels of serum IgE, increasing the likelihood of false positive sIgE results. With this kind of control group, we found the specificity of the IMMULITE 2000 method was as good as the UniCAP.
The methods of diagnosing IgE mediated food allergy, either by skin prick tests or by assaying for serum sIgE, are usually very sensitive but less specific, demanding confirmation of the results by means of oral challenge tests 1. This has led several groups to try to determine the decision point levels of sIgE with a higher positive predictive value for clinical reactivity 3-5. In a study involving a sample of children with atopic dermatitis, Sampson et al 9 proposed an optimal decision point of 32 kU/L for cow's milk allergy. In this population at this decision point level, the test showed a positive predictive value of 95 %, a sensitivity of 51 % and specificity of 98 %. In a subsequent work by the same group, this decision point was applied to another population of children referred for evaluation of food allergy, where only 61 % had atopic dermatitis. In this clinically different population, the sensitivity of the test decreased to 34 % and a better performance was obtained by using a lower decision point of 15 kU/L (57 % sensitivity, 94 % specificity). In a recent prospective study of a group of children with cow's milk allergy, Garcia-Ara et al 10 showed that the sIgE levels, which were predictors of clinical reactivity, increased with age. This suggests that age must also be taken into consideration when establishing sIgE cutoffs. All these data strongly suggest that the decision point levels of sIgE predictive of clinical reactivity are population specific 6.
It was not our intention to establish our own cutoff points in this study because our study population was not large enough; rather, we were interested in comparing the behavior of the two methods at the cutoffs established by other authors. We focused on two different decision points, both obtained using the UniCAP, in two different populations. The decision point proposed by Sampson (15 kU/L) was obtained in a population of older children, most of whom presented with atopic dermatitis. 3 The other, proposed by Garcia-Ara et al, was somewhat lower (2.5 kU/L), and was obtained in a population of infants with immediate-type symptoms 4. The different characteristics of the two populations could explain the different cutoffs obtained by the two groups.
In our study, we obtained the same performance with both methods by using different cutoff levels. Accordingly, the decision points seem also to be specific to the method. The main consequence is that the results obtained by the two methods should not be used interchangeably, since the same result could have a different meaning, depending on the method by which it was obtained.
Our study has shown IMMULITE 2000 3gAllergy to be a reliable method for detecting cow's milk allergy and as adequate as UniCAP in the diagnosis of CMA. Since decision levels of sIgE can depend on population, age, allergens, diseases and in vitro methods, each clinic must define the allergen cutoff for their own population. Care must be taken in avoiding a generalization of the use of the published cutoffs. The comparable clinical performance for milk allergy reported in this study indicated that either of the two methods can be used to obtain the population, age, disease and site-specific decision cutoff levels.
Correspondence:
Sara Prates
Serviço de Imunoalergologia
Hospital Dona Estefânia
Rua Jacinta Marto
1169-045 Lisboa
Portugal
Phone: + 351213126653
Fax: + 351213126654
E-mail: saraprates@netcabo.pt