INTRODUCTION
Nowadays, allergic diseases have an increased prevalence, which shows urban predominance in the Western world. Sensitisation to pneumoallergens develops when potential patients are exposed to environmental allergens over certain threshold level; the maintenance or worsening of the allergic disease depends on the continuous exposure to these allergens. The most relevant allergens found in the city of Barcelona are the house dust mites (HDM). As the only aetiologic treatments are specific immunotherapy and eradication of the allergens responsible of the allergic symptomatology, we have performed a study to assess the clinical improvement and the level of quality of life after the application of environmental control measures and the acaricide Frontac®.
HDM play an important role in the aetiology of perennial allergic rhinitis and asthma (1, 2). The symptoms of perennial allergic rhinoconjunctivitis are the same as those of seasonal allergic rhinoconjunctivitis, but nasal blockage is usually more intense; the symptoms are chronic and persistent, and the patients may present secondary complaints such as mouth-breathing, snoring, sinusitis or «a permanent cold» (3-5). Nevertheless, the patients with perennial allergic rhinoconjunctivitis are usually troubled not only by nasal/eye symptoms but also by the heavy impact on their health-related quality of life. The patients are limited in their inability to perform everyday activities, mental concentration is impaired, associated symptoms such as headaches are troublesome, practical things such as remembering to carry a handkerchief and repeatedly blowing the nose are a nuisance, sleep is impaired, social interaction is limited, and there is an impact on emotional well-being (6, 7).
The outdoor and indoor allergic environment has changed in most countries within the last decades and has increased the risks of allergic diseases. The microclimate of the modern house with fitted carpets, soft furnishings, central heating, double-glazing, and often with poor ventilation has provided the ideal habitat for HDM. When possible, environmental control measures for indoor allergens must be applied even if their efficacy is not complete, as they may generally improve the patient's condition and reduce the need for pharmacological treatment. Two major strategies have evolved: allergen avoidance/inactivation and mite population control by extermination. Thus, HDM avoidance can be attempted by reducing the amount of HDM itself, by reducing indoor humidity, by the application of acaricides and by the use of mite-proof covers (4). Nevertheless, even when an allergen source is removed from a patient's environment, the benefit may take several weeks or months to be perceived by the patient (8).
The aim of the present open, prospective, observational, multicentric study was to investigate over a period of one year (1999-2000) the effectiveness of a new acaricide-containing aerosol of esbiol/benzyl benzoate/piperonyl butoxide/2-phenylphenol (Frontac®) combined with the application of standard environmental control measures in the habitats of patients suffering from allergic respiratory pathology related to HDM and living in the city of Barcelona and surrounding area. Special interest was given to the analysis of the evolution of the patients' quality of life by using the Rhinoconjunctivitis Quality of Life Questionnaire (RQLQ) together with the analysis of clinical symptomatology. A secondary aim was to assess the impact of these measures on the antigenicity of house dust as assessed by the guanine content of the collected samples.
METHODS
Patients
The inclusion criteria were the following: 1) patients of both genders between 18 and 75 years of age; 2) residence in the city of Barcelona or in the nearest villages (< 10 km) in order to homogenise the environmental conditions; 3) presence of symptoms of perennial allergic rhinoconjunctivitis (sustained rhinorrhea, sneezing, nasal obstruction, nasal and eye itching and conjunctival watering) (4), with or without mild bronchial asthma, as defined by the American Thoracic Society (9); 4) positive skin prick tests to HDM (weal diameter ≥ 3 mm after 15 min of exposure); 5) normal baseline lung function (FEV1 > 80 %), and 6) serum specific IgE ≥ class 2 radioallergosorbent (RAST) test (Cap System). The exclusion criteria were the following: 1) immunotherapy during the period of study; 2) presence of pets in the patient's house; 3) patients without vacuum cleaner; 4) intolerance to some of the compounds present in the acaricide-containing aerosol; 5) patients who were participating in another study four or less weeks before inclusion, and 6) pregnancy. All patients provided their written informed consent.
Protocol
The present study was a prospective, open, observational, multicentric study that assessed the effect of an acaricide-containing aerosol of esbiol/benzyl benzoate/piperonyl butoxide/2-phenylphenol (Frontac®, Química Farmacéutica Bayer S.A., Barcelona, Spain), combined with the application of standard environmental control measures, on several clinical and environmental parameters. Esbiol and benzyl benzoate have a well-known acaricide effect (10) whereas piperonyl butoxide increases the acaricide effect of esbiol (11). Moreover, 2-phenylphenol is a fungicide that provides a complementary action by killing the fungi used by HDM as food, thus decreasing the HDM colonisation (12). The period of study was 12 months, with one initial visit and 4 quarterly follow-up visits. At the beginning of the study, the homes of 55 patients were visited by an allergology-trained registered nurse. The places where HDM may develop (mattresses, sofas, rugs, fitted carpets, curtains, etc.) were established and vacuumed for 2 min/m2. All vacuum applications were performed using the same vacuum cleaner. The acaricide-containing aerosol was further applied to this material susceptible to be colonised by HMD and these places were vacuumed again 1-2 hours later. The rooms were ventilated between the two vacuum applications; vacuum cleaners with microfilters (High Efficiency Particulate Air (HEPA)) were recommended to the patients. These procedures were performed monthly in absence of the allergic patient and standard environmental measures were used during all the 12-months period (13, 14).
The amount of HDM antigen was semi-quantitatively estimated using the Acarex(test (Merck Farma y Química, Barcelona, Spain). Guanine was extracted from raw dust with potassium hydroxide, and the amount present was estimated by the intensity of colour change on the test strip. For the test, it was presumed that the guanine thus identified came from mite faeces and that guanine levels were related to the antigenicity of the dust (15,16). The results were rated according to a 4-colour scale: negative = 0, slightly positive = 1, moderately positive = 2, and highly positive = 3 (16, 17). The patients were selected in a correlative way after compliance of inclusion criteria and after in vivo and in vitro tests characteristic of the speciality (18). The patients were asked to answer a questionnaire on their socio-economic status and housing conditions. Anterior rhinoscopy, basal rhinomanometry, and spirometry (only in patients with asthma) were performed at each quarterly visit. The severity of the symptoms was scored nominally (0 to 3) as follows: none = 0, mild = 1, moderate = 2, and severe = 3. In vivo (nasal provocation and skin prick test to HDM, Dermatophagoides pteronyssinus [Dp] and Dermatophagoides farinae [Df]) and in vitro tests (determination of total [fluoroimmunoassay] and specific IgE to Dp and Df [Pharmacia CAP System, Uppsala, Sweden]) were performed at baseline and at the end of the study. The patients' quality of life was assessed using the Rhinoconjunctivitis Quality of Life Questionnaire (RQLQ) (6), translated into Spanish. All adverse events were documented in order to control the toxicity of the aerosol throughout the study. Moreover, the patients were asked to self-assess the subjective change in their disease by grading the severity compared to that measured during the previous visit, as follows: better, same, or worse.
Statistics
The database was created using the SPSS software v.10.0 for Windows, and the statistical analyses were performed using the SAS software v. 6.12 for Windows. Efficacy and toxicity analyses were performed on an «intention-to-treat» basis. First, the normality of the distribution was assessed using the Kolmogorov-Smirnov test and the homogeneity of variances was assessed using the most restrictive of either Bartlett's or Cochran's test. Nominal qualitative variables were assessed using the chi-square analysis or Fisher's exact test for low frequency cases. Ordinal qualitative variables or quantitative variables with a log-normal distribution were assessed using the Friedman repeated measures ANOVA (analysis of variance), whereas quantitative variables with normal distribution were assessed using a one-way repeated measures ANOVA test. Differences between data collected only at baseline and at the final visit were assessed using the Wilcoxon signed rank or the Student t tests, as appropriate. All analyses were performed using an alpha level of 5 %. The data shown are the mean ± SD.
RESULTS
Characteristics of the patients
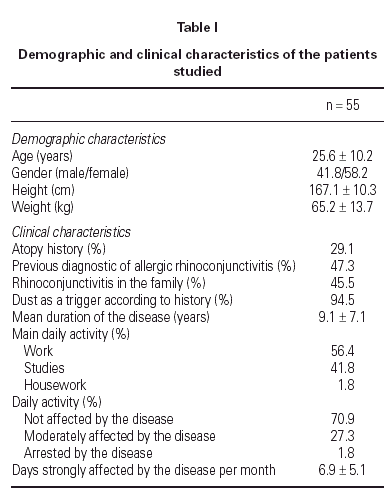
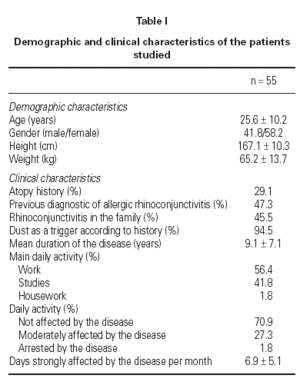
The demographic and clinical characteristics of the patients at the beginning of the study are summarised in table I. Data were collected from: 55 case report forms (CRFs) completed by the researchers (100 %); 49 CRFs completed by specialised auxiliary nurses (89.1 %) that included the RQLQ, the guanine test results and the adherence to environmental measures, and 29 diaries completed by the patients. The most severe baseline symptoms were moderate nasal obstruction (1.98 ± 0.73) and sneezing (1.90 ± 0.75). Other nasal symptoms found were mild to moderate rhinorrhea (1.67 ± 0.73) and itching (1.44 ± 0.75). Moderate oedema and intranasal inflammation was found in 67.7 % of analysed patients, but nasal polyps were not found. Moreover, mild to moderate eye symptoms were found at baseline: itching (1.15 ± 0.85), watering (1.12 ± 0.78) and erythema (1.00 ± 0.89). Pulmonary symptoms related with asthma (dyspnoea, wheezing) were found to be not relevant and baseline FEV1 was found to be normal in most patients. Total IgE levels in serum were abnormal in 51.6 % of the measurements (277.49 ± 281.23 IU/ml). IgE anti-Dp levels in serum were 30.2 ± 33.2 KU/ml and IgE anti-Df levels were 28.4 ± 31.5 KU/ml. Seven patients continued receiving immunotherapy during the period of study.
Characteristics of the homes and cleaning practices
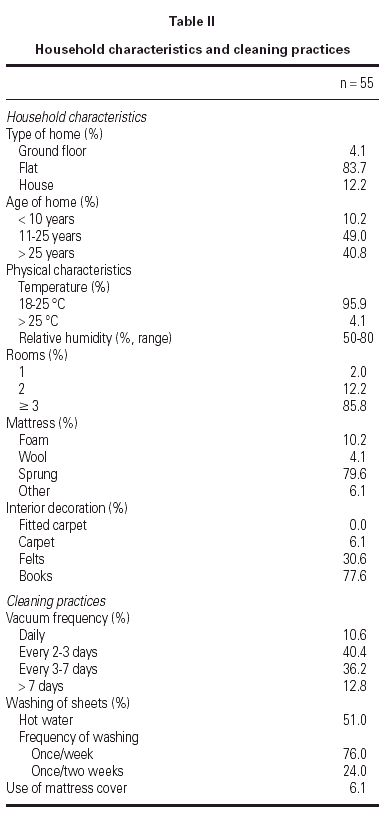
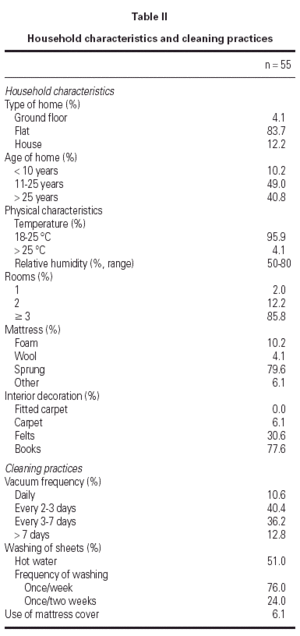
The types of housing and cleaning practices are summarised in table II. Most patients lived in flats older than 10 years with more than 3 rooms. Sprung mattresses were predominant and carpets, fitted carpets or mattress covers were scarce. Cleaning practices were common: 87.2 % of the patients vacuumed at least once at week. Compliance of standard environmental measures was assessed: at the beginning of the study, only 46.6 % of the patients regularly applied the measures proposed, whereas at the end of the study this percentage increased significantly to 90.0 % (p < 0.001).
Clinical symptoms
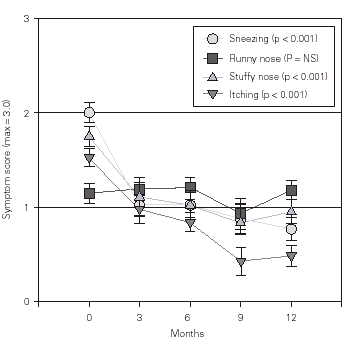
There was a significant decrease in all nasal symptoms scores, except for nasal secretion (fig. 1). Moreover, oedema and intranasal inflammation decreased significantly after 12 months (25.8 % of the patients, p < 0.001). With regard to pharyngeal symptoms, the daytime dry cough score but not the night-time dry cough score improved significantly (p < 0.03). The respiratory symptoms scores did not change during the study. Subjective self-assessment showed that 37.0 % of the patients improved after 3 months of the study whereas this percentage increased to 68.4 % after 12 months.
Figure 1.--Changes in the score of nasal symptoms. The score was rated from 0 (none) to 3 (severe). NS = non-significant. The data shown are means ± SEM.
Quality of life of patients
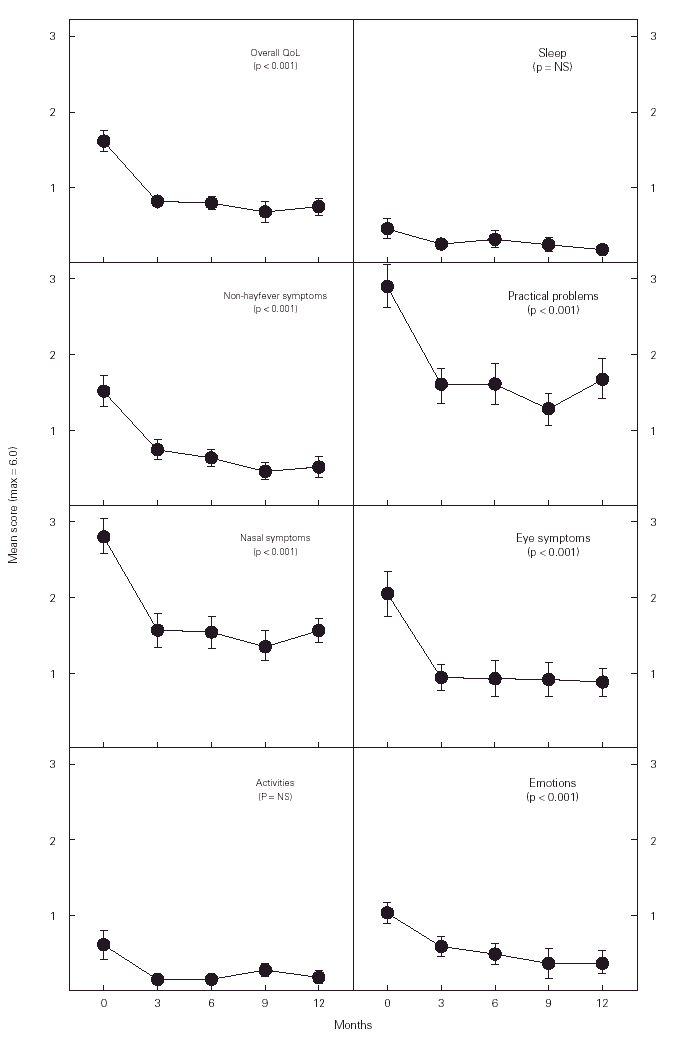
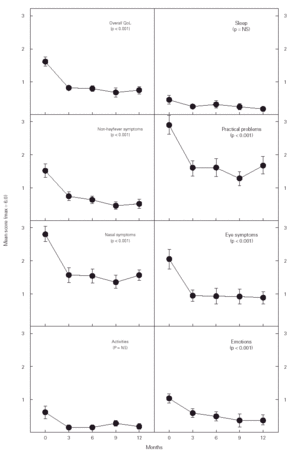
The overall quality of life (QoL) score improved significantly during the study period (fig. 2). The mean score decrease found was 0.86 (CI 95 %, 0.54-1.17). Five of the seven domains included in the questionnaire decreased significantly: non-hayfever symptoms (mean decrease of 0.97, CI 95 % 0.56-1.39); practical problems (1.25, CI 95 % 0.63-1.88); nasal symptoms (1.23, CI 95 % 0.70-1.76); eye symptoms (1.14, CI 95 % 0.62-1.67), and emotions (0.64, CI 95 % 0.26-1.01). These decreases were found during the first 3 months and later remained stable at lower levels than at baseline.
Figure 2.--Changes in the quality of life (QoL) of subjects studied. The score was rated from 0 (not disturbing) to 6 (extremely disturbing) except for emotions where 0 (never) and 6 (all the time). NS = non-significant. The data shown are means ± SEM.
Antigenicity of HDM
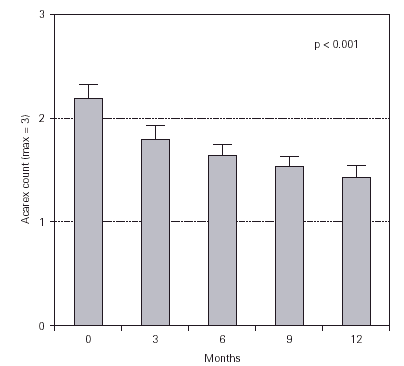
The content of guanine in dust samples decreased significantly from 2.17 ± 0.75 to 1.43 ± 0.68 (fig. 3). Overall, the presence of HDM in dust samples decreased in 70 % of the analysed homes.
Figure 3.--Changes in the antigenicity of dust samples measured from guanine content (Acarex® test). The data shown are means ± SEM
Toxicity of Frontac®
No adverse events or toxic effects were recorded during the one-year study period.
DISCUSSION
The results of the present study suggest a clinical benefit derived from the use of the tested acaricide-containing aerosol, combined with standard environmental measures, in the homes of patients suffering from perennial allergic rhinoconjunctivitis. The efficacy of an acaricide is measured as the capacity to kill adult mites but also larvae and eggs. Clinical improvement is a consequence of reduced exposure to allergen following removal by vacuum cleaning of mites killed by the acaricide. If vacuum cleaning is not done properly, large numbers of dead mites remain in the dust; they gradually disintegrate and more allergen-bearing, aerosolisable particles are generated. Therefore, although this was an open uncontrolled study it is relevant that the allergic patients achieved a satisfactory symptom control and improved their quality of life even after one year of follow-up. Long-term studies are advisable: other authors have previously found improvement in the clinical variables of patients with HDM-allergic asthma only after one year of study but not after 4 months (19). Moreover, studies performed on a one-year basis allow the detection of possible seasonal modifications.
Rhinoconjunctivitis symptoms are not life-threatening but have detrimental effects on the physical, psychological and social aspects of the patients' lives, and can decrease their quality of life significantly (20). Therefore, it is becoming increasingly accepted that both health-related quality of life (HRQoL) assessments and clinical measurements are needed to obtain a complete evaluation of the health status of the patients with allergic rhinitis. HRQoL data can be used to identify optimal therapeutic regimens but also to suggest practice guidelines for allergic disorders (21). The reliability of HRQoL data is highly dependent on the tool with which they are gathered: the RQLQ used here is one of the first disease-specific instruments validated to measure HRQoL in rhinoconjunctivitis (6). The RQLQ measures both physical and emotional function, reflects areas of function important to patients with rhinoconjunctivitis, provides reproducible scores amenable to statistical analysis and when the clinical status is stable it is responsive to clinical changes, even small ones. In the present study, we have found higher baseline scores for nasal/eye symptoms but also for practical problems (i.e., need to blow nose repeatedly; need to rub nose/eyes, and inconvenience of having to carry handkerchiefs). The decreases found in these and other RQLQ domains reflected that symptomatic improvement was accompanied by an improved quality of life of the patients.
An important environmental control step is to minimise HDM exposure in mite-allergic patients in order to reduce the morbidity of the allergic disease. The use of chemical acaricides supplements the HDM avoidance measures, as chemical acaricides are effective in reducing mite allergen levels (22-25). Both the wide range of allergic problems that can be caused by HDM and the ways an effective acaricide can improve them have been demonstrated in several clinical studies: some used aerosols, foams or powders containing benzyl benzoate in the home of patients with allergic rhinitis (26, 27), although most of them were performed in the homes of patients with allergic asthma (28-33). Other studies were performed using esbiol and piperonyl butoxide in the homes of patients with allergic rhinitis or asthma (34). Relapse of symptoms following exposure to untreated dusty environments was observed in many patients, thus confirming that the improvement reported was due to the removal of HDM allergen (26). Nevertheless, other studies with benzyl-benzoate failed to show significant differences versus placebo (30, 35) or environmental control alone (17) in the homes of allergic asthma patients. Therefore, the clinical relevance of the use of chemical acaricides under the variable conditions and the great diversity of HDM inhabitable niches found in the homes of allergic patients remains controversial. In many cases, products with double activity (i.e. acaricide and fungicide) are recommended (36).
The present study is the first that reports the acaricidal effectiveness and the subsequent clinical benefit of the use of Frontac® combined with environmental control for human beings. The results obtained with the Acarex® test showed the effective duration of the acaricide activity of Frontac®, since guanine content of dust samples decreased even after a year; this is a relevant finding, as an initial dramatic decrease of mite load by mechanical or chemical means is commonly followed by rapid recolonization (36) and vacuum-cleaning alone does not reduce mite numbers significantly (37). Besides, we confirmed the lack of toxicity of the chemical acaricide used in this study; this supports its use in long periods, a common procedure in the treatment of HDM-related allergic diseases.
In conclusion, the present study suggests a clinical benefit of short-term (3-months) and long-term (1-year) use of the new chemical acaricide tested, together with standard environmental measures, in the homes of patients suffering from perennial allergic rhinoconjunctivitis, with or without bronchial asthma. After one year, the severity of rhinoconjunctivitis symptoms decreased and the quality of life of patients improved while no toxicity of the acaricide was found. Nevertheless, the present results should be treated with caution; its use as an useful and additional preventive treatment of allergic patients must be confirmed in future randomised, controlled clinical trials comparing this efficacy with that of environmental control alone or other acaricides, but also by measuring changes in HDM-allergen levels using specific immunoassays.
ACKNOWLEDGEMENTS
We would like to thank Gemma Medina (ATS, Institut Universitari Dexeus) for her help in the management of the study and Vicente Alfaro (Biomedical Systems Group S.A) for his help as medical writer.