Invariant natural killer T (iNKT) cells play complex functions in the immune system, releasing both Th1 and Th2 cytokines. The role of iNKT cells in human asthma is still controversial and never described in severe therapy-resistant asthma in children. The objective of this work was to analyse iNKT frequency in peripheral blood of children with severe therapy-resistant asthma (STRA), compared to children with milder asthma and healthy controls.
MethodsChildren with asthma (n=136) (non-severe and STRA) from a referral centre and healthy controls (n=40) were recruited. Peripheral blood mononuclear cells were isolated, stained with anti-CD3 and anti-iNKT (Vα24Jα18), and analysed through flow cytometry. Atopic status was defined by measuring specific IgE in serum. Airway inflammation was assessed by induced sputum.
ResultsChildren with asthma presented an increased frequency of CD3+iNKT+ cells (median 0.38% IQR 0.18–1.9), compared to healthy controls (median 0.26% IQR 0.10–0.43) (p=0.025). Children with STRA also showed an increased frequency of iNKT cells (1.5% IQR 1.05–2.73) compared to healthy controls and non-severe asthmatic children (0.35% IQR 0.15–1.6; p=0.002). The frequency of iNKT cells was not different between atopic and non-atopic children. In addition, iNKT cells were not associated with any inflammatory pattern of induced sputum studied.
ConclusionOur data suggests that iNKT cells play a role in paediatric asthma, which is also associated with the severity of disease, but independent of the atopic status.
Asthma is a public health problem that affects 300 million people worldwide and it is a major health problem in children, with a high prevalence and morbidity.1,2 As a complex and heterogeneous inflammatory disorder, asthma is characterised by airflow obstruction, airway oedema, mucus hyper-secretion and bronchoconstriction.3 Over the last decades, many treatments for asthma have become available to decrease inflammation and to control the disease.4 Inhaled corticosteroids, usually associated with long-acting beta-2 agonists, are the preferred treatment for asthma.5 However, some patients do not respond to high-doses of corticosteroids, and are then diagnosed with severe therapy-resistant asthma (STRA). In children, STRA has been reported as an important clinical challenge in many referral centres.6 Children with STRA present diverse inflammatory patterns in the airways with an unrevealed mechanism of pathogenesis.7 Paediatric STRA seems to be characterised by bronchial remodelling and variable airway eosinophil counts, with an absence of Th2 cytokines in most patients, reduced IL-10 production, and increased IL-33 expression.8,9
Studies with mice models of asthma have shown that an innate subset of T cells, the invariant natural killer T cells (iNKT), plays a role in the pathogenesis of asthma.10–12 They are called invariant due to a very restricted TCR use, consisting of Vα14 and Jα18 in mice and Vα24 and Jα18 in humans.4,13,14 Activated iNKT cells release large amounts of both type 1 and type 2 cytokines, with critical roles in inhibiting or exacerbating allergic responses.4,13 In humans, the role of iNKT cells is unclear, with studies showing contradictory results.15 In addition, the expression of iNKT cells has never been reported in children with STRA. Consequently, the aim of the present study was to evaluate the expression of iNKT cells from peripheral blood of children with STRA, comparing with a group of patients with milder disease, and healthy controls.
Materials and methodsSubjectsMild asthma and healthy control groupsChildren aged 8–14 years with mild-to-moderate asthma, and a control group of healthy children, were recruited from a large cross-sectional study from public schools in 2011. Healthy children should have no history of any chronic disease and be free of respiratory symptoms over the previous four weeks. Children with asthma had to present with a history of wheeze and use of asthma medications in the previous 12 months, and a medical diagnosis of asthma. The diagnosis of asthma was then confirmed at the study centre by one of the investigators, based on the criteria of the Global Initiative for Asthma (GINA) guidelines, with the presence of recurrent symptoms suggestive of asthma (wheezing, cough, dyspnoea, and chest tightness) and airflow limitation or bronchodilator reversibility in lung function. Severity status of asthma was also confirmed by the 2009 GINA guidelines criteria used at that time during the recruitment period. Patients were then classified according to symptoms, as intermittent, mild persistent, and moderate persistent asthma.16
Severe therapy-resistant asthma groupChildren with STRA were recruited from a reference tertiary centre. The criteria for the diagnosis of STRA were: persistent disease despite treatment with high doses of inhaled corticosteroids (≥800mg/day of budesonide or equivalent), associated with long-acting beta-agonists, with or without leukotriene receptor antagonist. Persistent disease was characterised by: (1) persistent symptoms (>3 months); (2) acute exacerbations (with ICU admission, at least two hospitalisations, or at least two courses of oral steroids over the previous 12 months); (3) persistent airflow obstruction following steroid trial; or (4) need for alternate-day or daily oral steroid to achieve control.17 Children with chronic diseases other than asthma, such as neurological disorders, congenital cardiac diseases or immunodeficiency were excluded. This study was approved by the Human Ethics Committee of Pontifícia Universidade Católica do Rio Grande do Sul (# 10/04978). Legal guardians and/or children read and signed an informed consent form.
Mononuclear cell isolationPeripheral blood mononuclear cells (PBMC) were purified from 10mL of whole blood using a Histopaque-1077 (Sigma, USA) gradient and frozen at −80°C. After thawing, 5×105 PBMC were stained with anti-CD3 APC (SK7) and anti-iNKT Vα24Jα18 FITC (6B11) (BD Bioscience®, USA). Cells were acquired on a FACS Canto II flow cytometer (BD Biosciences®, USA). Cytometric data were analysed using FlowJo software (FlowJo®, USA).
Atopic statusChildren atopic status from the group of mild asthma and healthy controls was defined by measuring specific IgE in serum through ImmunoCAP test (Pharmacia Diagnostics AB). For both groups, the allergens analysed were: Dermatofagoides pteronyssinus, Blomia tropicalis, Dermatofagoides farinae, Periplaneta Americana, dog, cat, fungi, pollen, grass and dust. Those who presented more than 0.35KU/L for any of the allergens were considered atopic. For the analysis between atopic and non-atopic children, only the groups of non-severe asthma with ImmnunoCAP test analysis was included. Children with STRA atopic status was defined by skin-prick test with the same allergen panel used in the ImmunoCAP tests, according to a well-established method and allergens (ALK-Abelló, Denmark). A positive test was defined by wheal allergen ≥3mm in the absence of a positive reaction to the negative control.
Induced and sputum processedSputum was induced based on protocols previously reported.18 Briefly, the procedure was initiated 15min after the administration of 200μg of inhaled salbutamol, through inhalation of saline solution concentration of 4.5%. If FEV1<70% of predictive value, a saline solution of 0.9% was used. The samples were considered adequate if the total and differential cell counts could be obtained from a sample with cell viability >50%, and with squamous cells <20%. The sample was then suspended in 0.5mL phosphate buffered saline (PBS) and then stained with May-Grumwald Giemsa. Differential cell counts were obtained after counting 400cells and the results were expressed as a percentage.18 Sputum samples were labelled as eosinophilic (eosinophils ≥2.5% and neutrophils <54%), neutrophilic (eosinophils <2.5% and neutrophils ≥54%) or mixed (eosinophils ≥2.5% and neutrophils ≥54%).19
Statistical analysisDemographic data were summarised using descriptive statistics. Continuous variables were shown through means and standard deviation, or median and quartile values. The significance of differences between groups was compared using Kruskal–Wallis test, followed by Dunn post hoc analysis, and Mann–Whitney U test was applied to non-parametric variables. A p value of less than 0.05 was considered significant for comparisons. Statistical analysis was performed on SPSS, version 16.0 (SPSS, Chicago, IL, USA).
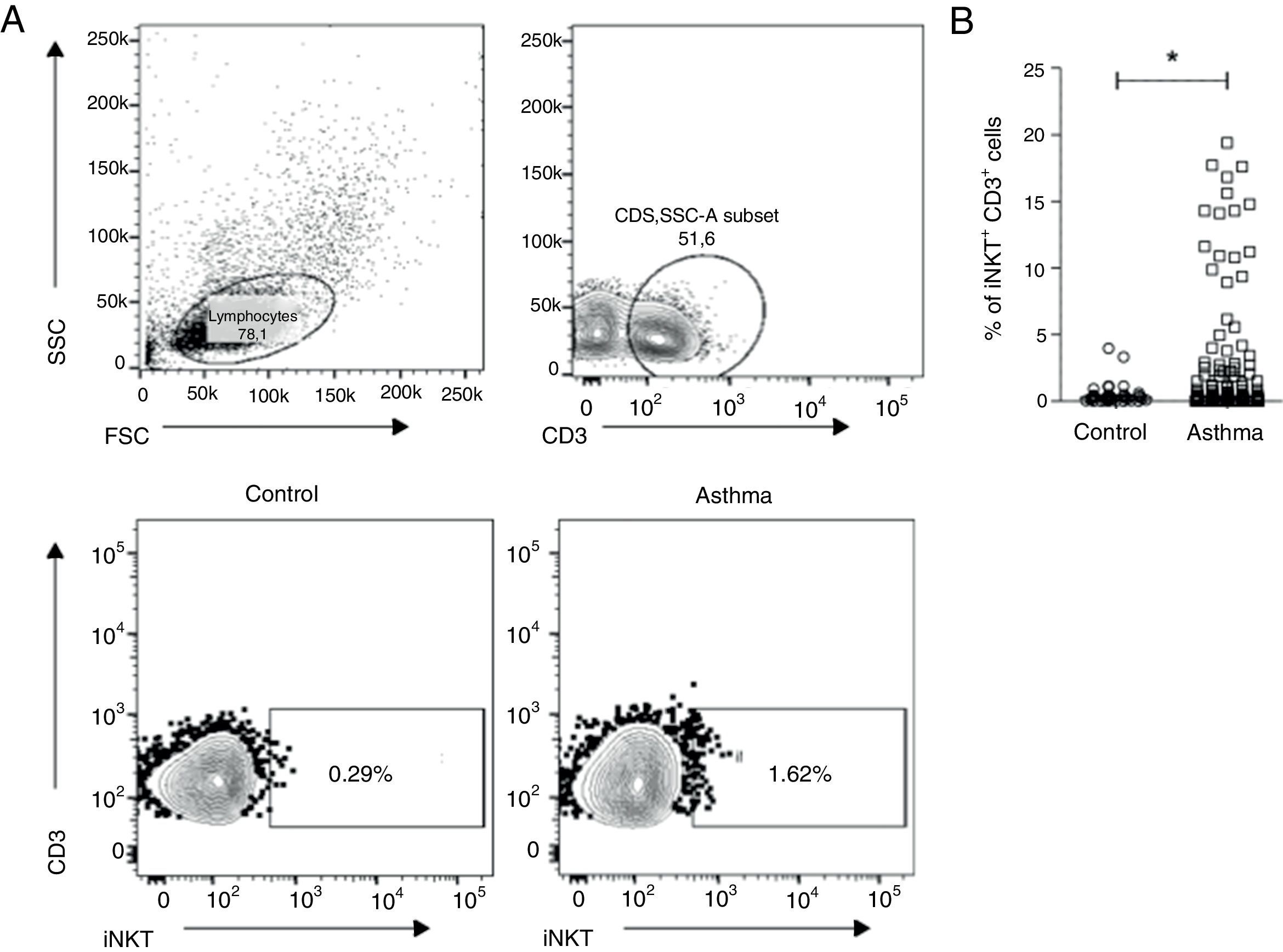
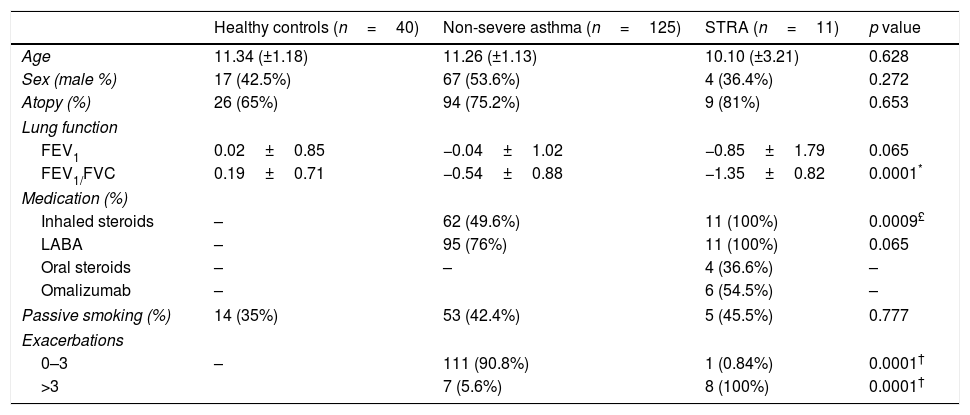
ResultsClinical characteristics of the studied subjects are shown in Table 1. Age, gender and atopic status were not different between the groups studied. Peripheral blood was collected from 136 asthmatic children and 40 controls. PBMC were isolated and stained with anti-CD3 and anti-TCR Vα24/Jα18 (iNKT). Fig. 1A shows the gate strategy used to analyse iNKT cells. Children with asthma presented a higher percentage of CD3+iNKT+ (median 0.38% IQR 0.18–1.9) compared to healthy controls (median 0.26% IQR 0.10–0.43) (p=0.025) (Fig. 1B).
Patients characteristics.
| Healthy controls (n=40) | Non-severe asthma (n=125) | STRA (n=11) | p value | |
|---|---|---|---|---|
| Age | 11.34 (±1.18) | 11.26 (±1.13) | 10.10 (±3.21) | 0.628 |
| Sex (male %) | 17 (42.5%) | 67 (53.6%) | 4 (36.4%) | 0.272 |
| Atopy (%) | 26 (65%) | 94 (75.2%) | 9 (81%) | 0.653 |
| Lung function | ||||
| FEV1 | 0.02±0.85 | −0.04±1.02 | −0.85±1.79 | 0.065 |
| FEV1/FVC | 0.19±0.71 | −0.54±0.88 | −1.35±0.82 | 0.0001* |
| Medication (%) | ||||
| Inhaled steroids | – | 62 (49.6%) | 11 (100%) | 0.0009£ |
| LABA | – | 95 (76%) | 11 (100%) | 0.065 |
| Oral steroids | – | – | 4 (36.6%) | – |
| Omalizumab | – | 6 (54.5%) | – | |
| Passive smoking (%) | 14 (35%) | 53 (42.4%) | 5 (45.5%) | 0.777 |
| Exacerbations | ||||
| 0–3 | – | 111 (90.8%) | 1 (0.84%) | 0.0001† |
| >3 | 7 (5.6%) | 8 (100%) | 0.0001† | |
Values are presented as mean and (±SD) or %.
PBMCs were collected from children with asthma (n=136) and healthy controls (n=40), and stained with anti-CD3 and anti-iNKT Vα24Jα18 to assess the presence of invariant natural killer T (iNKT) cells by flow cytometry. (A) Representative flow cytometric analysis of iNKT cells performed in children with asthma and healthy controls. (B) Frequency of CD3+iNKT+ cells. Normality of the samples was tested using Kolmogorov–Smirnov, and Mann–Whitney (U) test was used for comparison of two groups.
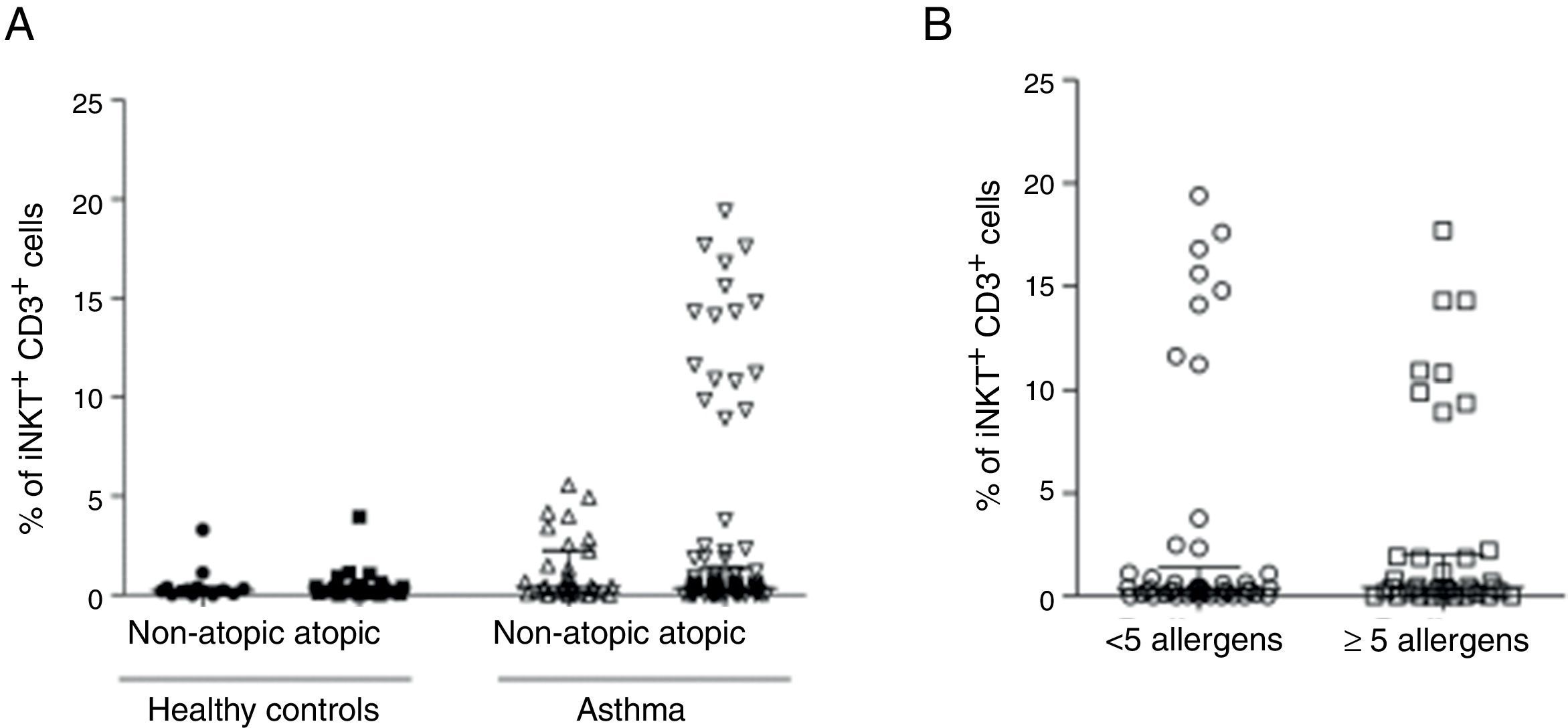
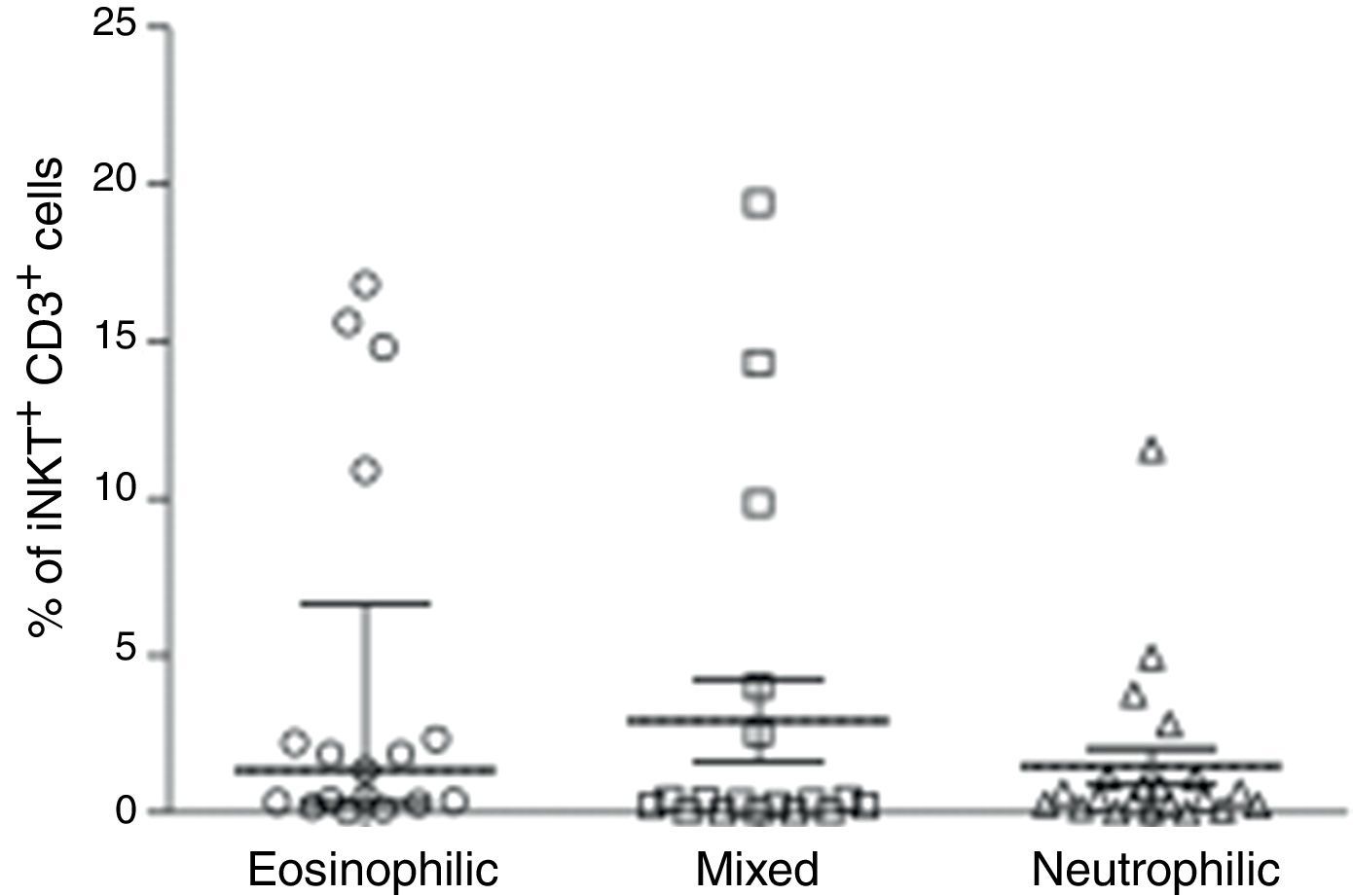
We then analysed whether iNKT cells were associated with atopy in this group of patients. The atopic status of asthmatic children used for this analysis was defined by measured allergen-specific IgE levels in serum. Hence, children with STRA were excluded from this analysis because the atopic status was defined by a different method (skin-prick test). The percentage of CD3+iNKT+ cells between children with atopic asthma (n=94) (median 0.33% IQR 0.13–1.3) and non-atopic asthma (n=31) (median 0.44% IQR 0.18–2.25) were not significantly different (Fig. 2A). However, we observed that some patients with asthma show iNKT frequencies five-fold greater than others. We then analysed the relationship between iNKT frequency and asthma allergen sensitisation, comparing patients highly sensitised (>5 allergens) with less sensitised (<5 allergens), although there was no significant difference between these two populations (Fig. 2B). iNKT cell frequencies were also compared between different patterns of inflammation in induced sputum of children with asthma, classified as eosinophilic, neutrophilic, mixed (eosinophilic and neutrophilic). iNKT cell numbers were not associated with any airway inflammatory pattern (Fig. 3).
PBMC were collected from atopic (n=94) and non-atopic (n=31) children with asthma, and atopic (n=26) and non-atopic (n=14) healthy controls. The frequency of invariant natural killer (iNKT) cells was evaluated after staining with anti-CD3 and anti-iNKT Vα24Jα18 by flow cytometry. (A) Frequency of CD3+iNKT+ cells. (B) Frequency of CD3+iNKT+ cells in highly sensitised (>5 allergens) children with asthma compared to less sensitised (<5 allergens) patients. Normality of the samples was tested using Kolmogorov–Smirnov, and Kruskal–Walis test followed by Dunn post-test was used.
PBMC were collected from children with asthma with different patterns of inflammation in induced sputum (eosinophilic (n=17), neutrophilic (n=21), mixed (n=18)). The frequency of invariant natural killer (iNKT) cells was evaluated after staining with anti-CD3 and anti-iNKT Vα24Jα18 by flow cytometry. Kruskal–Walis test followed by Dunn post-test was used.
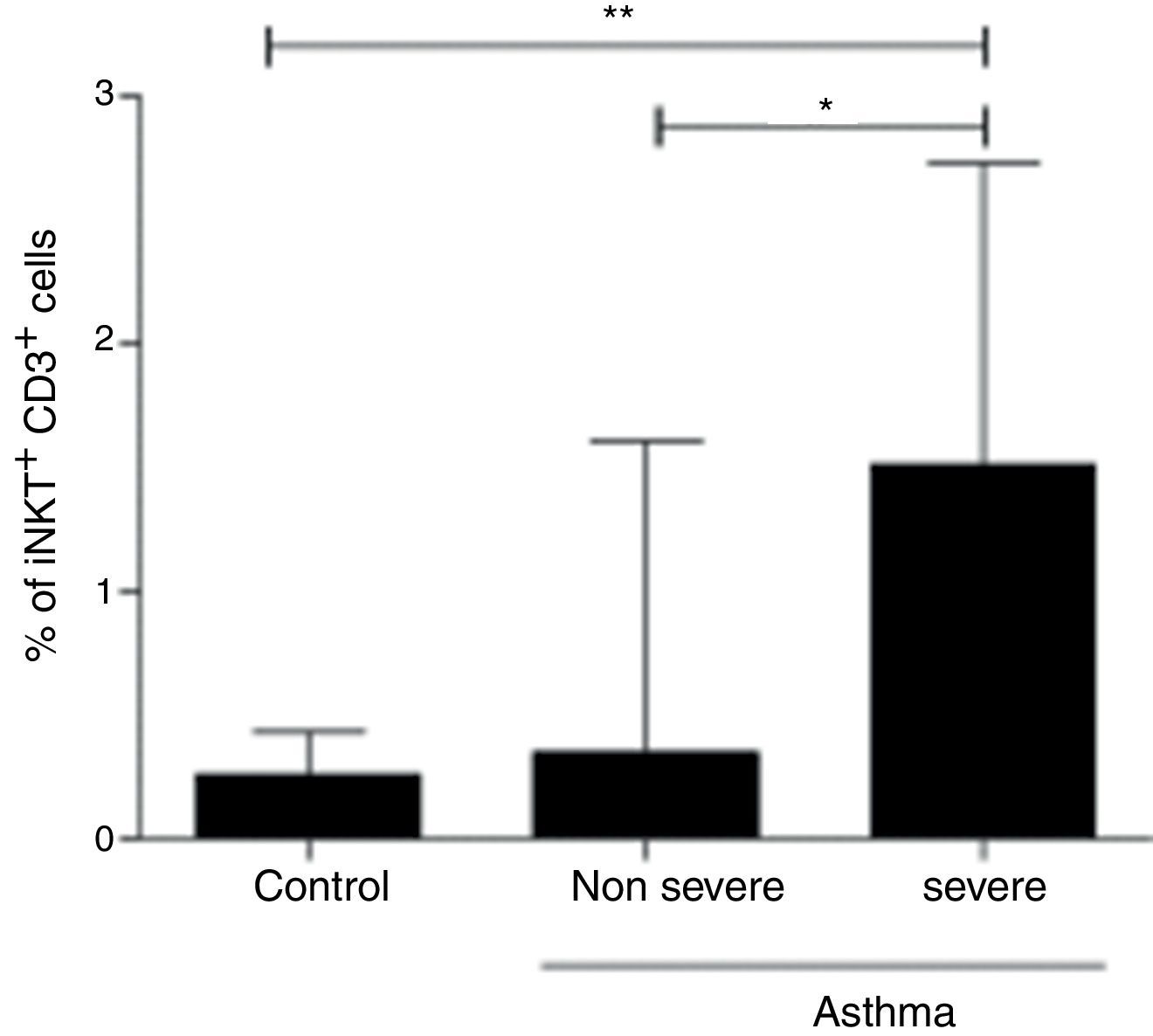
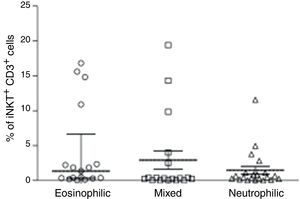
In order to assess the association of iNKT cells with the severity of asthma, we compared patients with non-severe asthma (n=125), children with STRA (n=11) and healthy controls (n=40). We found a significant increased frequency of iNKT cells in children with STRA (median 1.51% IQR 1.05–2.7) compared to non-severe patients (median 0.35% IQR 0.15–1.6) (p=0.002) (Fig. 4). In addition, the frequency of iNKT cells was significantly higher in children with STRA compared to healthy controls (Fig. 4).
PBMC were collected from children with non-severe asthma (n=125), children with STRA (n=11), and healthy controls (n=40). The frequency of invariant natural killer (iNKT) cells was evaluated after staining with anti-CD3 and anti-iNKT Vα24Jα18 by flow cytometry. Kruskal–Walis test followed by Dunn post-test was used.
Asthma is a complex and heterogeneous disease, with many cells rather than only Th2-type cells recognised to contribute to the pathogenesis of the disease. It has been previously demonstrated that iNKT cells are required in allergen-induced airway inflammation and bronchial hyper-responsiveness in a murine model of asthma.20 iNKT cells represent a bridge between innate and adaptive immunity, recognising glycolipid antigens attached to a non-classical MCH class I (CD1d).21 Similarly to conventional T cells, iNKT cells are selected in the thymus, based on their T-cell receptor (TCR), which is conserved and functions as a pattern recognition receptor.22,23 Activated iNKT cells rapidly secrete cytokines and chemokines, which activate dendritic cells (DCs), macrophages, NK cells, T cells and B cells.24 In addition, iNKT cells produce perforin and granzyme, similar to NK cells.
The role of iNKT cells in human asthma has been scarcely studied and showed controversial results. One previous study showed that fewer than 2% of the T cells from airway biopsy, bronchoalveolar lavage and sputum were iNKT cells in patients with asthma.25 In contrast, other study reported that more than 60% of CD4+ cells in bronchoalveolar lavage of patients with asthma were iNKT cells, while iNKT cells were not observed in healthy subjects.26 In our study, children with asthma presented increased frequency of iNKT on peripheral blood. Our results are in consonance with the study of Carpio-Pedroza et al., in which iNKT cells were increased in peripheral blood of children with asthma during exacerbation.27 However, in our study we further showed that children with STRA present higher levels of iNKT cells compared to non-severe asthma and healthy controls. It has been shown that iNKT cells were increased in bronchoalveolar lavage of adults with severe poorly-controlled asthma.12 STRA in childhood is still poorly understood, and clinical, pathophysiological and molecular features have to be further explored.28 Previous studies have focused on the mechanisms of disease in children with STRA, suggesting that a low Th2 immune response pattern and increased expression of IL-10 and IL-33 may be involved in this severe phenotype. Our results suggest that iNKT cells play a role in the orchestration of asthma and severe phenotypes in children. A humanised antibody specific to iNKT cells for in vivo depletion has been proposed, which could be an alternative to better study the role of these cells in future asthma treatment strategies.29 In addition, intestinal microbiota is suggested to be involved in modulation of lungs iNKT cells and asthma susceptibility.30 In germ-free mice iNKT cell accumulation in the lung and susceptibility to asthma can be rescued by colonisation with standard microbiota,31 suggesting a new approach to asthma intervention.
iNKT cell frequency was analysed in samples from peripheral blood. This factor could be considered a limitation. Samples from lower airways have been considered the ideal site for studying the immune response in asthma. Nevertheless, due to the requirement of invasive procedures to perform bronchial biopsy or bronchoalveolar lavage, ethical issues usually preclude this alternative in children, particularly in milder disease. However, on the other hand, peripheral blood could be a more attractive sample site alternative for clinically assessing potential biomarkers of disease in the future, which justifies the attempt to include peripheral blood in studies of immune response in paediatric asthma. Another issue to be discussed is the different therapies used by the children with asthma. In particular, corticosteroids and omalizumab (anti-IgE) are expected to be closely associated with the immune response. Our sample is not large enough to statistically analyse any association between the type of medications used and the number of iNKT cells. Hence, this is one limitation of the study, and we cannot rule out any influence of medications on our results, particularly those administered systemically (oral steroids and omalizumab).
In conclusion, our results show that iNKT cells are increased in children with asthma, particularly in the STRA phenotype. Advances in the comprehension of iNKT cell immune functions in asthma could lead to future clinical benefits, either as a biological marker or as an alternative target for novel therapies.
Ethical disclosuresConfidentiality of dataThe authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Protection of human subjects and animals in researchThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Conflict of interestAll authors have no conflict of interest to declare.
This study was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) Grant number 557291/2009-7. Liana Antunes received a scholarship from PUCRS.