Despite advances in research, the pathophysiology of food allergy has not yet been fully elucidated. IL-10 has both a pro- and anti-inflammatory effect on the development of food allergy and in order to understand its different immune-modulatory effects the factors that influence the inflammatory microenvironment need to be taken into account. Specific single nucleotide polymorphisms of the IL-10 gene seem to confer an increased risk of developing food allergy, but to date there is a substantial lack of genome- wide association studies regarding the genetic and epigenetic underpinnings of the disease. Special interest has been drawn to the development of allergen-specific regulatory CD4+CD25+ T-cells secreting IL-10 in the immunotherapy of allergic diseases. In addition, a distinct population of human tolerogenic dendritic cells (DC), DC-10 seems to hold great potential and could potentially serve as a therapeutic tool to improve the management of food allergy.
In recent years, intensive research on food allergy has provided the evidence that the pathogenesis of allergic reactions to food antigens is far more complex than previously considered. New insights include gene- environmental interactions that seem to play an important role in the development of food sensitization. Since genetic predisposition does not always lead to the development of allergic disease, research has also focused on the influence of epigenetic regulation in genetically susceptible individuals. Special interest has been drawn to the impact of early microbial colonization on the development of innate and adaptive immune pathways. Despite these advances, food avoidance remains the mainstay of the management in food allergy, and immunotherapy aiming at establishing early oral tolerance is largely the realm of clinical trials with promising results.
Undoubtedly, the complex pathways leading to T- helper cell type 2 (Th2) immune responses against food antigens cannot be attributed to a single driving force. It is, however, of special interest that of all cytokines, interleukin 10 (IL-10) is the only one that can both promote and downregulate Th2- dependent allergic responses and this review shall focus on the existing evidence of the unique role of IL-10 in the development of food allergy.
Why IL-10 is different compared to other cytokinesThere is an increasing body of evidence suggesting that the ultimate effects of IL-10 vary substantially depending on the experimental content and the cell types under investigation. IL-10 is a V-shaped homodimer with a molecular weight of 37 kDa. The human IL-10 contains 178 amino acids with a 160- amino acid mature segment and an 18 -amino acid signal sequence. It seems that the regulation of its expression is complex and delicately controlled at both transcriptional and post- transcriptional level.1–4 IL-10 and IL-10 receptor (IL-10R) deficiencies caused by loss-of-function mutations in the genes encoding IL-10 or IL-10R are rare primary immunodeficiencies resulting in severe dysregulation of the immune system and affect patients with severe early onset inflammatory bowel disease resistance to conventional immunotherapy often requiring allogenic hematopoietic stem cell transplantation (HSCT).5
IL-10 was originally named cytokine synthesis inhibitory factor; as a product of T helper (Th) type 2 cells, it shows the ability to inhibit T-helper 1 activation and T- helper 1 cytokine production.6 Over the years, it has been demonstrated that in addition to Th2 cells, there is a wide variety of haematopoietic cells producing IL-10, including macrophages, monocytes, dendritic cells (DCs), B cells, regulatory T cells (Tregs), Th1 cells, CD8 + T cells and Th 17 cells.7 Special interest has been drawn to the key role of IL-10 on T-cell development and differentiation by inhibiting the activation and maturation of macrophages and dendritic cells. However, its biological role is not confined to the antigen presenting cells, as IL-10 exerts an enhancing effect on B cells, mast cells, granulocytes, keratinocyte growth differentiation and natural killer (NK) cell proliferation and activation.1,8
The complex interplay between the genetic regulation, the wide cellular sources and the pleiotropic effect of IL-10 has made it challenging to fully elucidate the molecular mechanisms that modulate the cell type-specific IL-10 expression. Therefore, research is focusing on genomic boundaries, epigenetic modifications and transcriptional/posttranscriptional regulators that seem to be the main factors governing the IL-10 expression in vivo in host responses to inflammation.9–12
The role of IL-10 in immune responses in food allergyIt has been well described that in order to understand why individuals develop food allergies, we need to elucidate the factors that disturb the innate surveillance system that promotes oral tolerance (OT). The development of OT is a complex process and is defined as the active suppression of specific immune responses to antigens first encountered in the gastrointestinal tract that requires intact physical barriers, digestive enzymes, gastric acid, and specialized cells in the GI tract (intestinal epithelial, dendritic and microfold cells).13,14
Maintaining homeostasis is one of the two phases of the immune response in OT and under homeostatic conditions, the tight junctions between the enterocytes prevent the paracellular passage of the antigens.15–17 It is important that this initial phase is not interrupted, given that the GI tract encounters a significant protein load up to 100gr daily.18 The second phase of OT aims at suppressing the immune responses to antigens, a process that is mediated by antigen- specific regulatory T cells (Tregs).19
Animal models have demonstrated that the role of Tregs in OT is crucial.20–22 In particular, the peripherally induced antigen- specific CD4+CD25+ Foxp3+ Tregs is the subtype of Tregs that governs oral tolerance.23 Mouse models have been used to show that after the generation of Tregs in the lymph nodes, they need to undergo local expansion in the gut to induce oral tolerance. On the contrary, depletion of specific forkhead box P3 (Foxp3) cells abolishes established oral tolerance.24 These findings are in keeping with the results of the group of Torgerson who demonstrated that the absence of functional Foxp3- expressing regulatory T cells secondary to a mutation in the Foxp3 locus is associated with a loss of peripheral tolerance and severe food allergy in humans.25
It has been well described that atopic disorders and elevated serum IgE levels occur in a number of primary human immunodeficiencies. The crossroads of autoimmunity and immunodeficiency become more evident in immunodysregulation, polyendocrinopathy and enteropathy, X linked (IPEX) syndrome that affects male subjects afflicted with a mutation in FOXP3. Patients with IPEX syndrome can present with severe atopic disease manifesting as eczema and food allergy with peripheral eosinophilia and increased IgE level.26–28 Autosomal recessive combined immunodeficiencies, such as the dedicator of cytokinesis 8 (DOCK8) deficiency, have also been reported to present as an IPEX- like syndrome with similar atopic manifestations.29 In Wiskott- Aldrich syndrome (WAS) patients demonstrate increased frequency of sensitization to food allergens and increased prevalence of clinically relevant food allergy in childhood. Experimental studies on WAS protein (WASP) deficient mice demonstrated that WAS deficiency limited to Foxp3+ Tregs resulted in a strongly Th-2 skewed inflammation of the small intestine which was exacerbated compared with that of complete Was −/− counterparts, indicating that WASP is required for Foxp3+ Tregs to exert selective control over Th2-type immune responses.30
Tregs seem to play a central role in the maturation of the immune system and the onset of immune-mediated diseases. Studies in pre-term and full-term infants showed similar or even superior Treg percentages and absolute counts to those in adolescents and adults signifying their implication in maternal-fetal tolerance.31 On the contrary, the deficit of Tregs seems to be a predisposing factor for food allergy onset in infants.32
In addition, a recent study focused on the role of dietary non-digestible, short-chain galacto-, long-chain fructo, and pectin-derived acidic oligosachharides (GFAs) showed that dietary GFAs enhance the Treg frequency in the mesenteric lymph nodes and mucosal IL-10 and transforming growth factor beta (TGF) transcription while suppressing the allergic effector response. This allergy-protective effect of the GFA diet was mediated by IL-10 and TGF-β in cow-milk- allergic mice.33 It has also been suggested that vitamin D improves the generation of allergen-specific Treg cell populations and therefore carries the potential to be considered as a safe therapeutic option in the management of food allergy. The group of Mabrouk et al. recently reported that vitamin D deficiency is common in children with IgE and non-IgE food allergy along with decreased number of CD4+CD25highFoxp3+IL10+ Treg cells that increased after the in vitro addition of vitamin D with increased Foxp3 and IL-10 expression.34
Two inhibitory cytokines, IL-10 and TGF- β, contribute to the ability of Tregs to induce a tolerant immune response to antigens.13 The suppression of the Th2-responses is mediated by the induction of allergen specific CD4+CD25+Foxp3 Tregs by dendritic cells (DC) that produce IL-10.35–37 It is thought that DC-derived IL-10 contributes to the reduction of inflammatory responses to allergens in subjects receiving immunotherapy as a result of the suppression of high-affinity IgE receptor Fc epsilon receptor I-dependent pro-inflammatory responses.38,39 IL-10’s key role as a regulatory cytokine includes the ability to decrease the synthesis of IgE and the survival of eosinophils.40,41 Low-dose oral tolerance against a potent milk allergen, beta lactoglobulin (BLG) has been shown to be mediated by BLG- specific/IL-10 secreting T regulatory type 1 cells (Tr1) in Peyer’s patches that inhibit the T-cell proliferative response in vitro and T-cell mediated inflammation in vivo.42,43
Interestingly, there is also evidence from animal models that IL-10 may have both pro- and anti- inflammatory effects during immune responses. It was recently described that IL-10 modulates the development of food allergy to the experimental food allergen ovalbumin by promoting the expansion of the mucosal mast cells and influencing their function during food allergy. These observations lead to the conclusion that perturbations of IL-10 activity may modulate the outcome of the allergic response in susceptible individuals.22 In accordance with these findings, the group of Geha et al. described the role of antigen presenting cells (APCs) and T cells in IL-10 skewing the Th response towards Th2 in a mouse model of allergic dermatitis, highlighting how IL-10 plays a key role in balancing the progression and resolution of allergic responses.44
In line with this, experimental studies on IL10-/- mice have shown that IL-10 is a key mediator of immune regulatory function. Resident enteric microbiota selectively induced intestinal IL-10- producing B cells through TLR2, MyD88 and PI3K pathways and these B cells reduced colonic T cell activation and maintained mucosal homeostasis in response to intestinal microbiota.45 The association of the gut microbiome with the immune mechanisms involved in food allergy was further investigated in a study aiming to demonstrate whether exposure of BALB/c mice to bifidobacterium bifidum TMC3115 in early life influences immunity and alleviates the risk of IgE-mediated allergies in adulthood. The study demonstrated that neonatal mice exhibited less increase in serum IgE levels induced by ovalbumin and significantly higher tumor necrosis factor- alpha (TNF- a) and IL-10 levels than in the control in adult mice.46
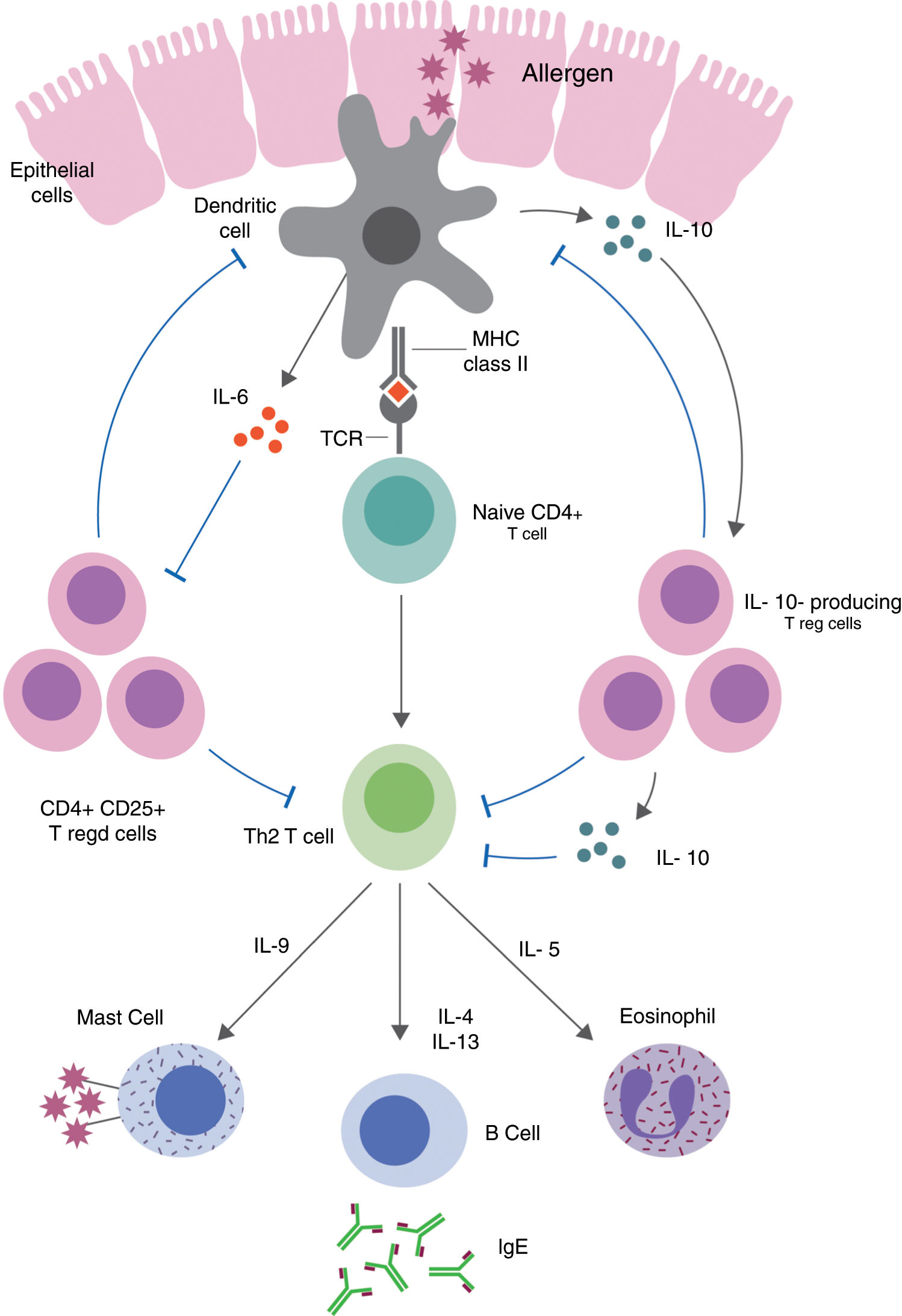
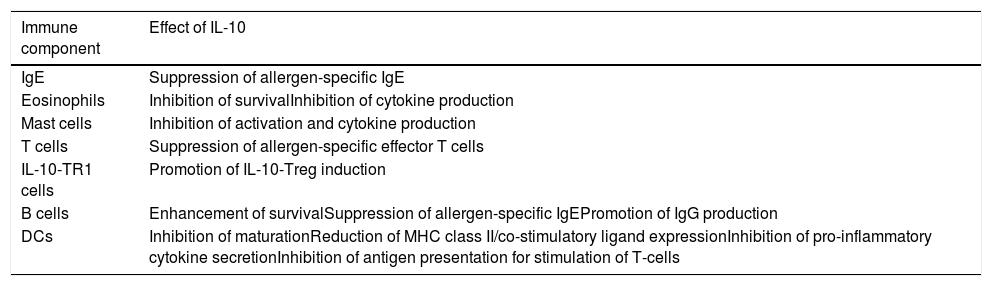
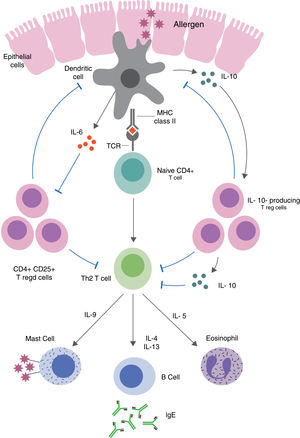
Table 1 summarizes the pleiotropic effects of IL-10 on specific IgE and different cell types involved in allergic diseases.47Fig. 1 demonstrates the immune pathways via which Tregs and IL-10 regulate food allergy.48,49 It is thought that in order to understand the different immune-regulatory effects of IL-10, we need to take into account the factors that influence the inflammatory environment (type of stimulus, genetic background of the cells used, cell culture conditions).22,50–53
Effects of IL-10 on the immune components involved in allergic diseases.
| Immune component | Effect of IL-10 |
|---|---|
| IgE | Suppression of allergen-specific IgE |
| Eosinophils | Inhibition of survivalInhibition of cytokine production |
| Mast cells | Inhibition of activation and cytokine production |
| T cells | Suppression of allergen-specific effector T cells |
| IL-10-TR1 cells | Promotion of IL-10-Treg induction |
| B cells | Enhancement of survivalSuppression of allergen-specific IgEPromotion of IgG production |
| DCs | Inhibition of maturationReduction of MHC class II/co-stimulatory ligand expressionInhibition of pro-inflammatory cytokine secretionInhibition of antigen presentation for stimulation of T-cells |
Treg-mediated regulation and suppression of food allergy. Defects in epithelial barrier can allow antigens to enter the lamina propria and trigger T cell activation. Induced allergen-specific Tregs skew the immune responses to a Th2-like phenotype and the production of pro-inflammatory cytokines via dendritic cell pathways.
Genetic testing in chronic diseases has been proven to be important as causal mutations can influence the course of the disease and help with the identification of patients at risk who might benefit from early interventions. Advances in sequencing and genotyping have shed light on the genetic mechanisms that contribute to the development of food allergy, with most studies focusing on candidate genes and the association with specific variants.54 Even though limited genetic research has been carried out in food allergy, a recent systematic review identified that the most reproducible genes for an association with food allergy to date include those of filaggrin (FLG), human leukocyte antigen (HLA) and interleukin 13 (IL13).55
The gene of IL-10 is located on the long arm of chromosome 1 (q31-q32). The production of the cytokine is associated with two single nucleotide polymorphisms (SNP) loci at positions G-1082A and C-627A that lie on the putative transcription factor-binding sites.56,57 A retrospective study in 220 Japanese children investigated the role of IL-10 as a regulatory cytokine of allergy and the results indicated that the genotype -627 A/A was associated with lower IL- 10 production and higher IgE level in the serum. The severity of food allergy could not be attributed to a single SNP, but it was the combination of SNPs, laboratory parameters and environmental factors that appeared to determine the severity of the disease.58 In the same population, another study did not observe an association between IL-10 -627AA SNP and the prevalence of food allergy59 and these findings were in keeping with the results of the Brown et al. group that however reported a significant difference in the gene expression level of the SNPs IL-10 (C-627A) between patients with positive radioallergosorbent test (RAST) versus RAST negative patients.60
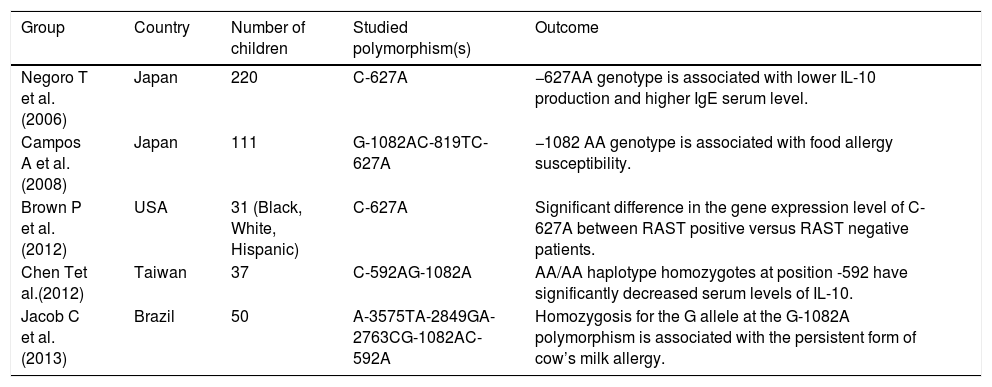
A statistically significant association between the -1082 AA genotype and the risk for food allergy was demonstrated by Campos et al.59 Five IL-10 SNPs (−3575A/T, −2489A/G, −2763A/C, −1082G/A, −592C/A) were studied in a cross-sectional study in Brazil that included children older than five years of age with IgE-mediated cow’s milk allergy (CMA). The presence of the G allele at G-1082A conferred a twofold risk of developing persistent CMA (p < 0.001) and the risk was even higher in the persistent CMA group compared with the tolerant group.61Table 2 shows the studies on IL-10 gene polymorphisms associated with food allergy in pediatric patients and their outcomes.
Studies on IL-10 gene polymorphisms associated with food allergy in pediatric patients.
| Group | Country | Number of children | Studied polymorphism(s) | Outcome |
|---|---|---|---|---|
| Negoro T et al. (2006) | Japan | 220 | C-627A | −627AA genotype is associated with lower IL-10 production and higher IgE serum level. |
| Campos A et al. (2008) | Japan | 111 | G-1082AC-819TC-627A | −1082 AA genotype is associated with food allergy susceptibility. |
| Brown P et al. (2012) | USA | 31 (Black, White, Hispanic) | C-627A | Significant difference in the gene expression level of C-627A between RAST positive versus RAST negative patients. |
| Chen Tet al.(2012) | Taiwan | 37 | C-592AG-1082A | AA/AA haplotype homozygotes at position -592 have significantly decreased serum levels of IL-10. |
| Jacob C et al. (2013) | Brazil | 50 | A-3575TA-2849GA-2763CG-1082AC-592A | Homozygosis for the G allele at the G-1082A polymorphism is associated with the persistent form of cow’s milk allergy. |
In the last few years, the focus of research into food allergy has shifted from a candidate gene approach to genome-wide association studies that provide the ability to discover novel disease candidate genes associated with moderate disease risk.62 Today there is still a considerable lack of knowledge regarding the genetic and epigenetic underpinnings of food allergy underscoring the need to explore the genetic variants specifically associated with clinical food allergy.63,64
Serum IL-10 levels in children with food allergyIt has been proposed that children with food allergy have reduced numbers of TGF-β-producing T cells in their intestinal mucosa and IL-10 producing T cells in their circulation. Food-sensitized patients with supportive skin tests and allergen-specific IgE measurements exhibited a lower proportion of T cells spontaneously secreting IL-10 without antigen stimulation compared with controls.65 Functional differences have also been shown in association with the IL-10 promoter gene polymorphisms and particularly the AA/AA haplotype homozygotes at position -592 had significantly decreased serum levels of IL-10 in children with food allergy.66 On the contrary, comparison of the IL-10 production in RAST positive and RAST negative patients showed a significant increase in IL-10 production in RAST positive patients indicating altering phenotypic responses in patients with various food allergies.60Table 2 summarizes the studies on IL-10 gene polymorphisms associated with food allergy in children and their outcomes.
The cow’s milk-specific T-cell response of donors with various allergic backgrounds was investigated by Tiemessen et al. and their results showed that activated cow’s milk specific T-cell clones of cow’s milk tolerant control subjects were characterized by the production of IL-10, suggesting that activated CD4 + T cells with high CD25 expression might contribute to the tolerogenic immune response towards an antigen through the production of IL-10.67 Determination of cytokine response to β-lactoglobulin (BLG) has also revealed significantly higher IL-10 levels during the tolerance phase with a parallel increase in BLG- IgG4 and BLG-IgG4/IgE ratio in children with cow’s milk allergy.68 In keeping with these findings, other groups have reported that the increase in IL-10 levels might be a useful tool in the diagnosis of food tolerance in previously food allergic patients.69,70 A recent double-blind placebo-controlled study of school-aged children with CMA who were treated with oral immunotherapy (OIT) showed that at the end of the OIT study the serum IL-10 and IL-6 were higher in the active group. However, even though there was a significant change in other biomarkers, the rise of IL-10 between the start and the end of OIT was not significant when the changes during the blinded and open OIT were analysed together for both groups.71
Immunotherapy of allergic diseases: does IL-10 have a key role to play?Based on the above observations, it would be reasonable to suggest that key steps in allergen-specific immunotherapy (AIT) should target the development of allergen-specific regulatory CD4+CD25+ T cells secreting IL-10 and/or TGF- β and the recovery of effector T- cells by cytokines from the tissue microenvironment.70 Oral immunotherapy with the administration of gradually increasing quantities of the antigen to which the patient is allergic achieves some level of desensitization, but the ability to induce long-term tolerance has not been routinely established yet.72 Clinical research and animal models have provided evidence about novel strategies and the promising use of adjuvants that can induce a quicker, more potent and longer- lasting AIT immune response.73,74
The therapeutic utilization of IL-10 against autoimmune diseases has not been proven effective and this has been attributed to the complexity of the immunomodulatory properties of IL-10 and the existence of feed-forward and feedback pathways that cannot be replicated by the simple offering of exogenous IL-10.75 To overcome these challenges, research has focused on dendritic cells (DCs) and specifically DC-10 that comprise a distinct population of human tolerogenic dendritic cells (tolDCs).
DC-10 have entered the clinical arena as inducers of antigen-specific T regulatory type 1 (Tr1) and emerging evidence indicates that IL-10- modulated mature DCs are the best-suited cells for tolDC-based therapies.76–78 IL-10 producing DC subsets have been shown to suppress allergen-specific Th2-responses via the induction of either Th1, Th1- like Tregs, CD4+CD25+Foxp3+ Treg or TR1 cells and therefore DC-based vaccination approaches hold great potential to improve the treatment of allergic diseases.79
ConclusionThe evolving understanding of the pathogenesis of food allergy emphasizes the need for the suppression of antigen specific T-cell responses to achieve effective prevention and treatment of the disease. Undoubtedly, IL-10 has a unique, pleiotropic role in regulating excessive immune responses, but it remains to be clarified if the dendritic cells with a pro-tolerogenic IL-10- producing phenotype can serve as the therapeutic approach that will lead to the treatment of allergic diseases.
Funding sourcesNone declared.
Conflict of interestThe authors have no conflict of interest to declare