The role of neutrophil gelatinase-associated lipocalin (NGAL) in childhood asthma remains unknown. This study aimed to measure the serum levels of NGAL in children with asthma and to investigate the correlation between NGAL and transforming growth factor beta 1 (TGF-β1), a good indicator of airway remodeling in children with asthma.
MethodsThis prospective, cross-sectional study was conducted on 75 children. Serum NGAL and TGF-β1 concentrations were measured by the ELISA method. Complete blood count, high sensitive C reactive protein (hsCRP), eosinophil cationic protein (ECP), and total serum IgE were investigated in the study population. Atopy in the asthma group was investigated using a skin prick test and specific IgE measurements.
ResultsForty-three asthmatic children and 32 healthy children were enrolled in the study. Total eosinophil numbers, white blood cell count, total serum IgE levels and ECP levels were significantly higher in the asthma group than in the control group (p<0.05). Similarly, serum TGF-β1 levels were significantly higher in children with asthma (p=0.012). The difference in NGAL levels between the groups was insignificant (p=0.268). NGAL levels did not show a significant correlation with total IgE, ECP, eosinophil numbers and TGF-β1 levels (p>0.05).
ConclusionAs a conclusion, while elevated TGF-β1 levels in children with asthma might be regarded as an indicator of airway remodeling, we did not find a similar prediction strength for NGAL. Further studies are required to better identify the role of NGAL in childhood asthma and to determine its potential use as a clinical marker.
Asthma is one of the most common chronic diseases, estimated to be affecting 300 million people worldwide, with an increasing prevalence especially among children. Asthma is a clinical diagnosis based on episodic symptoms and variable airways obstruction. It is also characterized by variable degrees of chronic inflammation and structural alterations in the airways. These structural alterations, generally called airway remodeling, encompass complex changes in composition, content, and organization of the various cellular and molecular constituents of the airway wall. The most important abnormalities are epithelial detachment, goblet cell hyperplasia, subepithelial thickening, hyperplasia and hypertrophy of airway smooth muscle, bronchial gland enlargement, angiogenesis, and alterations in the extracellular matrix component.1–3
Neutrophil gelatinase-associated lipocalin (NGAL) is a 25kDa glycoprotein first identified as a matrix protein of specific granules of human neutrophils.4 NGAL is secreted by neutrophils4 and other cells such as respiratory5 and intestinal6 epithelial cells, vascular endothelial cells,7 adipose tissue,8 macrophages9 and tubuli cells in the kidneys.10 The role of extracellular matrix proteins in the process of airway remodeling is well known. Matrix metalloproteinase-9 (MMP-9) and NGAL have been shown to be particularly associated with lung capacity and clinical severity. Several studies performed on patients with asthma, pulmonary emphysema, chronic obstructive pulmonary disease (COPD) revealed increased MMP-9 and NGAL levels in bronchoalveolar lavage (BAL) fluid samples, probably as a result of structural alterations in the airways.11–15 Additionally, a recent study reported significantly higher plasma NGAL levels in COPD patients than in control patients.16
The role of NGAL as a potential marker of disease severity in childhood asthma remains unknown. Research involving invasive bronchoscopy techniques in young children is limited; hence studies have focused on systemic markers indicating the possible structural alterations in the airways. In the present study we investigated the association between serum NGAL levels and clinical and laboratory parameters in children presenting with asthma. Additionally, we investigated the correlation between NGAL and transforming growth factor beta 1 (TGF-β1), a good indicator of airway remodeling in children with asthma.17,18
Materials and methodsStudy subjectsThis prospective, cross-sectional study was conducted on 75 children, 43 of whom suffered from asthma presenting with wheezing to the Fatih University pediatric allergy outpatient clinic and 32 of whom were healthy controls. The asthma group included children of ages ≤6 years, with at least four wheezing attacks during the previous year, and ages >6 years who were diagnosed clinically and functionally according to GINA criteria.2 Children of similar age and gender with no history of allergic disease or wheezing were selected as the control group. Healthy children were chosen among those being brought for routine controls. Those with chronic diseases (e.g. malnutrition, anatomic malformation of the respiratory system, chronic lung disease, heart disease, gastro-esophageal reflux disease, cystic fibrosis) were excluded from the study.
Venous fasting blood samples were collected into Vacuette tubes (Greiner Bio-One, Monroe, NC) and centrifuged at 3000×g for 15min at 4°C. Serum samples were stored at −80°C for not more than 6 months. Levels serum of TGF-β1 was measured by the ELISA (enzyme linked immunosorbent assay) method using a ELX-800 system (RayBiotech, Norcross, GA, US). Serum NGAL concentrations were determined with Biovendor Human Lipocalin-2/NGAL ELISA kit (BioVendor GmbH, Heidelberg, Germany). The limit of detection for this assay was 0.02ng/mL with total imprecision (as CV) <8% at concentrations of 23.63–68.19ng/mL. The antibodies used in this ELISA are specific for human lipocalin-2. Complete blood count was investigated with the LH-780 system (Beckman Coulter Diagnostics, Image 8000, Brea, CA, USA). Levels of high sensitive C reactive protein (hsCRP) were measured by turbidimetric assay method using a Roche P 800 modular system (Hitachi, Tokyo, Japan). Levels of eosinophil cationic protein (ECP) were measured by the chemiluminescent assay method using an Immulite 2000 systems (Simens, Llanberis, Gwynedd, UK). The eosinophil counts were measured by LH-780 system (Beckman Coulter, Mervue, Galway, Ireland). Levels of total serum IgE were measured by the ECLIA (electrochemiluminescence) method using a ELX-800 system (DIAsource, Nivelles, Belgium).
Atopy in the asthma group was investigated using a skin prick test (SPT) and specific IgE (sIgE) measurements. A test was considered positive if the SPT results demonstrated a wheal with a mean diameter of at least 3mm greater than that of a saline control. Each child was tested with a core battery of allergens (e.g. dust mite, cockroach, cat, dog, mold, grass, tree, weed, milk, egg, peanut) and a clinic-specific battery of locally relevant allergens (ALK Abelló, Hørsholm, Denmark). Spirometry (Vmax encore; VIASYS Healthcare Inc., Conshohocken, PA) and bronchodilator reversibility were defined as greater than a 12% or 200ml change from baseline FEV1. These parameters were measured according to the GINA criteria.2
Statistical analysisData analysis was performed using Statistical Package for the Social Sciences (SPPS) for Windows, version 16.0 (SPSS Inc., Chicago, IL, United States). All the continuous values were presented as mean±standard deviation (SD). The categorical values were presented as n (%). The homogeneous distribution of the data was evaluated using the Kolmogorov–Smirnov test. Homogeneity of variance was evaluated by the Levene test. In the comparisons of the groups, Student t-test was used. The chi-square test was used for categorical variables. Correlation between the independent parameters was investigated by bivariate (Pearson) correlation analysis. A p-value of less than 0.05 was considered as statistically significant.
The study was initiated upon approval by the Local Ethics Committee of Fatih University in accordance with the Helsinki Declaration. The written informed consent of the parent(s) of each subject was also obtained before the study.
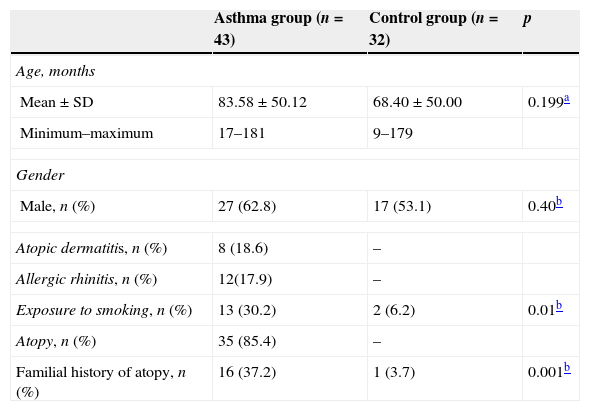
ResultsForty-three asthmatic children (mean age: 83.58±50.12 months; 27 male) and 32 healthy children (control group) (mean age: 68.40±50.00 months; 17 male) were enrolled in the study. Mean ages and gender distribution were similar between the groups (p>0.05). The demographic characteristics of study population are presented in Table 1.
Demographic and laboratory characteristics of study population.
| Asthma group (n=43) | Control group (n=32) | p | |
|---|---|---|---|
| Age, months | |||
| Mean±SD | 83.58±50.12 | 68.40±50.00 | 0.199a |
| Minimum–maximum | 17–181 | 9–179 | |
| Gender | |||
| Male, n (%) | 27 (62.8) | 17 (53.1) | 0.40b |
| Atopic dermatitis, n (%) | 8 (18.6) | – | |
| Allergic rhinitis, n (%) | 12(17.9) | – | |
| Exposure to smoking, n (%) | 13 (30.2) | 2 (6.2) | 0.01b |
| Atopy, n (%) | 35 (85.4) | – | |
| Familial history of atopy, n (%) | 16 (37.2) | 1 (3.7) | 0.001b |
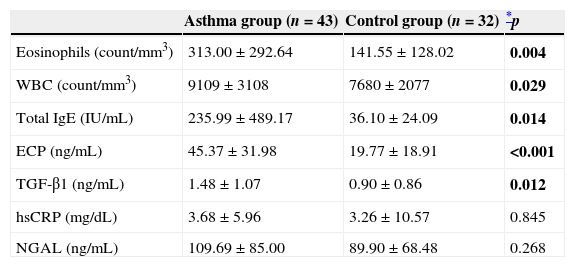
Total eosinophil numbers, white blood cell (WBC) numbers, total serum IgE levels and ECP levels were significantly higher in the asthma group than in the control group (p=0.004, p=0.029, p=0.014, and p<0.001; respectively). Similarly, serum TGF-β1 (ng/mL) levels were significantly higher in children with asthma (1.48±1.07, 0.90±0.86; p=0.012). Serum hsCRP levels, measured to exclude possible co-morbid infections, were similar between the groups (p>0.05). The difference in NGAL (ng/mL) levels between the groups was insignificant (109.69±85.00, 89.90±68.48; p=0.268) (Table 2).
Comparison of laboratory parameters between asthma and control groups.
| Asthma group (n=43) | Control group (n=32) | *p | |
|---|---|---|---|
| Eosinophils (count/mm3) | 313.00±292.64 | 141.55±128.02 | 0.004 |
| WBC (count/mm3) | 9109±3108 | 7680±2077 | 0.029 |
| Total IgE (IU/mL) | 235.99±489.17 | 36.10±24.09 | 0.014 |
| ECP (ng/mL) | 45.37±31.98 | 19.77±18.91 | <0.001 |
| TGF-β1 (ng/mL) | 1.48±1.07 | 0.90±0.86 | 0.012 |
| hsCRP (mg/dL) | 3.68±5.96 | 3.26±10.57 | 0.845 |
| NGAL (ng/mL) | 109.69±85.00 | 89.90±68.48 | 0.268 |
ECP, eosinophil cationic protein; hsCRP, high sensitive C reactive protein; NGAL, neutrophil gelatinase-associated lipocalin; TGF-β1, transforming growth factor beta 1; WBC, white blood cells.
Date are given mean±SD.
Children from the asthma group were classified into subgroups based on disease severity (12 mild intermittent, 26 mild persistent, 5 moderate persistent). None of these children presented with severe asthma. We found similar NGAL levels between the subgroups. While NGAL levels did not show a significant correlation with total IgE, ECP, eosinophil numbers and TGF-β1 levels (p>0.05), a weak correlation was noted between NGAL and hsCRP levels (p=0.015, r=0.292).
DiscussionAsthma is a disease of airway inflammation characterized by hyper-responsiveness and airway obstruction that lead to symptoms such as cough and wheezing. The remodeling process observed following chronic inflammation and airway obstruction in asthmatic patients may lead to structural alterations in the airways which in turn may result in a decrease in the lung functions and resistance to medical therapy.2 Data from the Tucson Children's Respiratory Study suggest that pulmonary function in children who ultimately have persistent asthma is normal at birth, with deficits acquired by as early as 6 years of age.19 Lung function data from a large cohort study, with follow-up from early childhood through age 35 years revealed that children with asthma had lower lung function during childhood and at age 35 years compared with that seen in cohort members without a history of childhood asthma.20 This remodeling process has been demonstrated in children with asthma by means of endobronchial biopsy samples.21,22 In addition to these data suggest that structural changes in the asthmatic children airway that might result in permanent lung function deficits occur early in the natural history of asthma. A good understanding of the remodeling process and structural alterations observed in children with asthma is important for the follow up and management of the patients. Since research involving invasive bronchoscopy techniques in young children is limited, studies have focused on non-invasive methods. NGAL, which is an extracellular matrix protein, has emerged as a potential marker of airway remodeling in children with asthma.
NGAL is characterized as a specific granule of human neutrophils and is known to be secreted from a number of tissues including the respiratory epithelial cells5 and macrophages.9 Current research is focused on the role of NGAL on various diseases including acute kidney injury, obesity, cancer, etc.23–25 Previous studies reported an increase in NGAL and MMP-9 levels in asthmatic patients with airway structural alterations.12–14 In a recent study, NGAL levels, when compared to matched controls, were shown to be higher in COPD patients presenting with notable airway structural alterations and with a significant increase in their extracellular matrix protein levels. Taken together, one may suggest NGAL as a potential predictor of airway remodeling.16 However, in the present study, we found similar serum NGAL levels in children with asthma and control subjects.
TGF-β1 is a profibrotic cytokine that is associated with airway fibrosis. TGF-β1 is believed to play an important role in most of the cellular biological processes leading to airway remodeling.18,26 Another study reported increased TGF-β1 concentrations and expression in children with asthma.17 Thus, TGF-β1 might be a key mediator of the airway remodeling in children with asthma. In this study although we found significantly higher serum TGF-β1 levels in children with asthma as compared to control subjects, the difference between NGAL levels were insignificant. This finding implies that NGAL is not a strong predictor of airway remodeling in children with asthma.
Our study has several limitations. First, the sample size was small. Second, the study group lacked of patients with severe asthma and third, concurrent bronchoalveolar examinations were missing. Higher serum TGF-β1 levels found in children with asthma as compared to control subjects indicates, however, an active airway remodeling and structural alteration process in these patients. Hence the absence of subjects with severe asthma in the study group may not actually be regarded as a substantial limitation.
To the best of our knowledge, this is the first study to investigate the role of NGAL in asthmatic children. As a conclusion, while elevated TGF-β1 levels in children with asthma might be regarded as an indicator of airway remodeling, we did not find similar prediction strength for NGAL. Further studies conducted on larger groups, preferably examined by means of bronchoscopic methods, are required to better identify the role of NGAL in childhood asthma and to determine its potential use as a clinical marker.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Conflict of interestThe authors have no conflict of interest to declare.
Funding/support: This study is supported by the Scientific Research Fund of Fatih University.