It seems clear that certain macrolide antibiotics exert anti-inflammatory and immune modulating effects beyond their purely antibacterial action, as has been demonstrated in a number of bronchial inflammatory disorders such as diffuse panbronchiolitis. Randomised, controlled clinical trials involving larger patient samples are needed to confirm whether these actions are of clinical relevance in application to asthma. On the other hand, the macrolide antibiotics have a long half-life, with a prolonged elimination interval, which appears to favour the development of resistances that persist over the long term, as in the case of azithromycin. Would the risk/benefit ratio of sustained low-dose macrolide use be justified, considering the risk of selecting resistant strains?
A number of questions must be answered before these drugs can be recommended in application to asthmatic patients: In which patients should they be used? Which drug or drugs would be most appropriate? What would the recommended dose be, and for how long should treatment be administered? What adverse effects can be expected?
In 1952, the group led by McGuire1 made an important contribution to antibiotherapy with the discovery of erythromycin. The authors obtained this antibiotic from a strain of Streptomyces erythreus, isolated from soil samples collected on the island of Paray (The Philippines). This drug, which must be administered in the form of acid-resistant salts and/or enteric formulas, is quickly degraded in the stomach.2,3
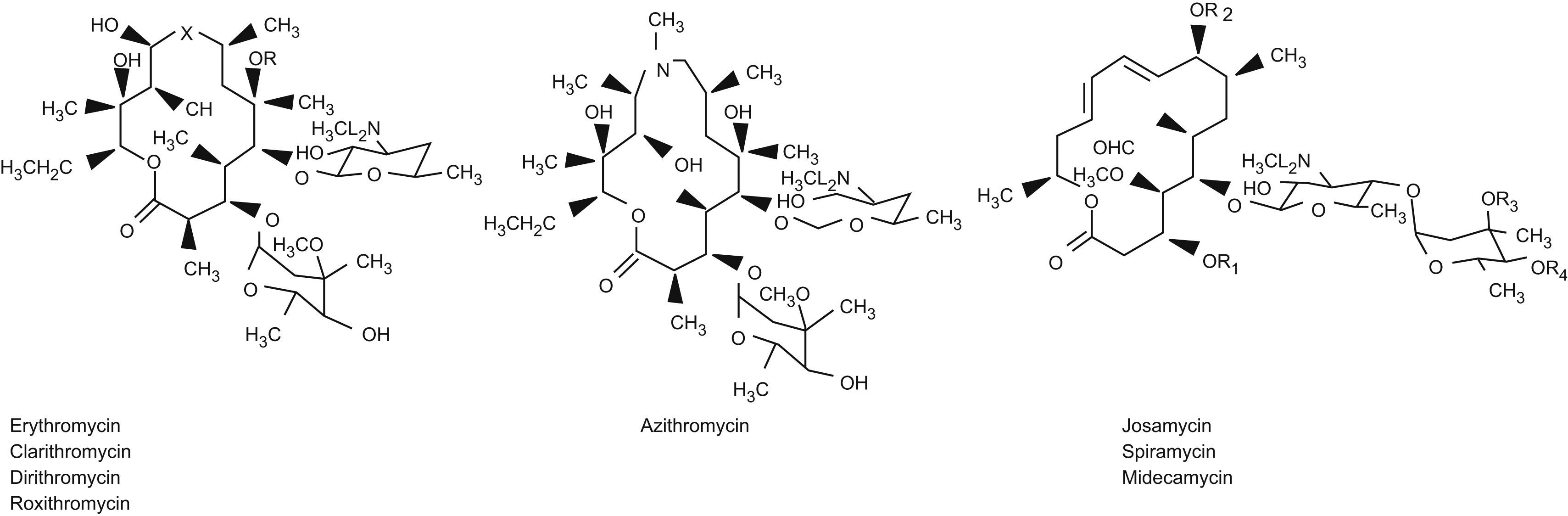
It represents a new family of antibiotics known as the macrolides, since their chemical structure includes a large lactonic ring to which one or more neutral (mediated by clonidase) or basic sugars (catalysed by amino-sugar deaminase) are linked via glycosidic bonds.
The number of carbon atoms conforming this macrocyclic structure allows classification of the different members of this drug family, according to whether the lactonic ring has 14, 15 or 16 carbon atoms. Over the years, new members have been added, resulting from modifications in the chemical structure, in the form of changes in the chemical groups that participate in the degradation process (Figure 1).
Thus, erythromycin results from substitution of the hydroxyl group in position 6 with a methoxy- group. In the case of roxithromycin, an ethyloxime radical is incorporated to position 9. Azithromycin in turn is obtained by incorporating a methyl- radical with a nitrogen atom in position 9, giving rise to a structure known as “azalide”, with a lactone ring of 15 carbon atoms, which confers increased activity against gram-negative bacteria.
The macrolides with a ring comprising 16 carbon atoms are more stable in acid medium than those with 14 or 15 carbon atoms (Table 1).
All macrolides act by inhibiting bacterial protein synthesis through binding with the 50S subunit of the 70S bacterial ribosome. More specifically, binding takes place with the RNA molecule of the 23S bacterial ribosome.4
The intimate mechanism by which this blocking of bacterial protein synthesis takes place differs among the different drug groups. In the group with 16 carbon atoms, the peptidyl transferase reactions of the ribosome are inhibited, while in the case of the group with 14 carbon atoms peptidyl transferase-RNA translocation is inhibited.5 In this way amino acid transfer during development of the new peptide is interfered with.
Bacterial resistance in turn occurs through methylation at 23S bacterial ribosome RNA level, which is transmittable via plasmids from one bacterium to another.
The spectrum of action of the macrolides encompasses gram-positive and gram-negative bacteria, actinomycetes, treponemes, mycoplasmas, chlamydias and rickettsias. The spectrum of action varies from one drug to another within the same group, as a result of the particular pharmacokinetic properties of each individual substance.
Macrolides are the treatment of choice in infectious processes caused by Legionella, Bordetella, Campylobacter and Helicobacter. This is partly because the drug concentrations within the cell cytoplasm are several times higher than in serum5 and the aforementioned bacteria develop at intracellular level. These antibiotics have a prolonged half-life, which delays elimination and produces very low but sustained drug concentrations in oropharyngeal secretions. These concentrations lack antibacterial efficacy and thus favour the selection of resistant strains.
A number of studies have compared the results of treatment with erythromycin, clarithromycin and azithromycin. Resistances were seen to develop at the end of therapy with all three drugs – although with the former two agents the patients had healed, and the resistances moreover disappeared over time.6 In contrast, in the case of azithromycin, the appearance of resistances is progressive, with the appearance of resistant microorganisms after 6 weeks of treatment in 90% of all patients, due to the prolonged presence of small and therapeutically ineffective concentrations of the drug in the host tissues.
Macrolide metabolisation7 takes place in the liver via the oxidative pathway mediated by the P450 cytochrome system.
Three cytochrome enzyme groups, CYP1, CYP2 and CYP3, conform the oxidative metabolic pathway of drug substances in humans. A number of isoenzymes have been described in these cytochrome groups (CYP1A2, CYP2C9, CYP2C19, CYP2D6, CYP3A4), accounting for the great majority of the observed drug interaction-related side effects.
The macrolides are able to induce their own liver-mediated biotransformation into nitrosoalkanes, via metabolic oxidation of the –N(CH3)2 group of the drug to the corresponding –NO group. The nitrosoalkanes in turn are metabolised by CYP3A4, with inhibition of the catalytic activity of this isoenzyme. This is the mechanism responsible for most of the macrolide pharmacological interactions. Azithromycin (a 15-carbon atom azalide) does not interact with the P450 cytochrome system, and thus has a lesser potential for interacting with other drug substances.
Susceptibility to infection among asthmatic patientsThere is abundant evidence of a special susceptibility among asthmatic patients to both viral and bacterial infections.
In effect, asthmatics have a greater risk of developing infection due to rhinoviruses, and the associated symptoms are moreover more intense and persistent than in healthy individuals.8 In this context, it has been shown that rhinoviruses infecting the bronchial epithelial cells of asthmatic patients replicate much more effectively than in healthy subjects, where replication proves very difficult.9
This observation was attributed to a strongly diminished production of IFN-β, and the cells of asthmatic patients were seen to recover a response similar to that observed in healthy subjects, upon administering IFN-β. Approximately 80% of all asthma exacerbations are caused by viruses, and of these, two-thirds correspond to rhinoviruses.10,11
The bronchial epithelial cell receptor for rhinoviruses is the ICAM-1 adhesion molecule, and the vulnerability of these cells in asthmatics seems to be related to the lesser production of interferon (IFN) in such individuals.12
The production of IFN-β in response to rhinovirus infection is diminished in the bronchial epithelium of asthmatic patients. This in turn limits the apoptotic capacity of these epithelial cells, thus extending their lifespan and increasing viral replication.9
Viral infection induces a host inflammatory response characterized by a predominantly neutrophilic infiltration, together with the presence of eosinophils, CD4+ and CD8+ cells, and mast cells—with an important increase in proinflammatory cytokines and chemokines (IL-6, IL-8, IL-16, eotaxin, RANTES, IP-10, etc.).13
Recently, an experimental model has been developed in mice that suffer virally induced exacerbations of asthma, thereby facilitating improved knowledge of the underlying mechanism involved.14
Exacerbations of asthma produced by bacteriaSome studies have described a relationship between viral infections in asthmatic individuals and the presence of atypical bacteria. Cunningham et al.15, in a group of asthmatic children, reported a strong association between the levels of anti-C. pneumoniae IgA and the frequency of asthma exacerbations The authors measured these antibodies in nasal secretions, and also detected the presence of viruses in 85% of the cases.
Another study in adults with asthma exacerbations seen in the emergency room found that 38% of the patients had serological evidence of the reactivation of C. pneumoniae infection, as well as an increased inflammatory response of the bronchial airways.16 Likewise in this case, viral detection proved positive in 76% of the subjects.
It is therefore of great interest to clarify whether viral infection induces reactivation of infections due to atypical bacteria, and whether these in turn favour the appearance of viral infections and hence of asthma exacerbations.
In the same way as stated for viruses, atypical bacteria also condition bronchial inflammation—inducing the secretion of cytokines on the part of nucleated cells in peripheral blood17 and alveolar macrophages.18
In turn, the bronchial epithelial cells also induce the expression of NTFα, IL-8, IFNγ and nuclear factor κβ (NF-κβ), as well as activation of the latter. In mice19, both M. pneumoniae and C. pneumoniae have been found to cause bronchial hyperresponsiveness (BHR) and inflammation.
On the other hand, it has recently been reported that asthma is a risk factor for invasive pneumococcal disease.20
Importance of M. pneumoniae and C. pneumoniae in patients with stable asthmaA number of studies have reported increased infection rates due to both M. pneumoniae and C. pneumoniae in adult asthmatics, compared with the healthy population. Martin et al.21, in a series of 55 adults with stable asthma and 20 healthy controls, found positive results for one or both of the mentioned bacteria in 56% of the asthmatic subjects, upon applying a polymerase chain reaction (PCR) technique to bronchial samples. M. pneumoniae was detected in 23 asthmatics (42%) and in only one healthy control (5%) (p<0.007). In the case of C. pneumoniae, seven patients proved positive (13%), versus none of the controls (p<0.04).
Similar results have been published by Brisccione22 in a population of adult asthmatics with healthy controls (patient couples), based on serial sampling of nasal secretions over a three-month period. This author detected a significantly higher frequency of M. pneumoniae in patients with stable asthma, and related this finding to a decrease in IFNγ production in response to stimulation by bacterial lipopolysaccharides (LPS).
It should be mentioned that these findings could possibly be even more notorious if the existing methods for detecting such germs were more sensitive, and thus more effective. In this sense, a review published by Johnston23 speculates upon the possible role of these microorganisms in the pathogenesis of asthma.
The results of the studies made in both adults16 and in the paediatric population24 regarding the presence of M. pneumoniae and/or C. pneumoniae infection, or of evidence of the reactivation of chronic infection by these germs, suggest that the reactivation of C. pneumoniae infection could be associated with an increased inflammatory response of the airways. It should also be mentioned that associated viral infection is detected in a large percentage of cases (up to 70–80%)—thus suggesting possible interactions between both types of microorganism and the local inflammatory response25.
Anti-inflammatory and immune regulating effects of macrolidesSince the first studies carried out in patients with diffuse panbronchiolitis (DPB)26, fundamentally by Japanese authors, we have become aware of the effects of several macrolide antibiotics upon different inflammatory response mediators—effects which have made it possible to modify the sombre prognosis of DPB. More recently, it has been shown that improvement in patients with DPB is independent of their genetic susceptibility to the disease.27
Azithromycin, a 15-carbon atom macrolide (azalide), has been found to be effective in children with asthma for reducing BHR and neutrophilic infiltration. Piacentini28, in a sample of 16 asthmatic children treated for 8 weeks with azithromycin or placebo, measured FEV1 at baseline and after treatment, and observed no significant changes. This author also assessed BHR through provocation with nasal hypertonic saline solution, establishing the corresponding dose-response slope, and detected a significant reduction in the azithromycin group, but not in the placebo series. In turn, he examined induced sputum cellularity and recorded a significant reduction versus baseline in the neutrophil count in the group of children treated with the antibiotic, while the placebo series showed no changes.
Previously, Tamaoki29, in isolated human bronchial tissue preparations, had demonstrated the inhibition of neurally mediated contraction with both roxithromycin and clarithromycin—probably involving the inhibition of exocytic acetylcholine release from the nerve endings.
At present, many studies have concluded that the macrolides exert anti-inflammatory and immune regulating actions in pulmonary inflammatory disorders such as asthma, cystic fibrosis, DPB, etc.
In this sense, both in vitro and in vivo data show these drugs to down-regulate prolonged inflammatory response; reduce airway mucus secretion30,31; inhibit the bacterial adhesion biofilm32; reduce the production of reactive oxygen species33,34; inhibit neutrophil activation and mobilisation with an acceleration of the apoptotic process35,36; and also block the activation of nuclear transcription factors.37,38 In turn, they exert a sustained suppressive effect upon cytokine secretion by the human bronchial epithelial cells39–41, inhibiting or activating kinase-regulated extracellular signaling.42,43
Mechanisms have also been proposed which include increases in the primary immune response, inhibition of epithelial cell-bacterial interaction44,45, modulation of the cytokine-mediated response pathways, and direct actions upon the neutrophils42.
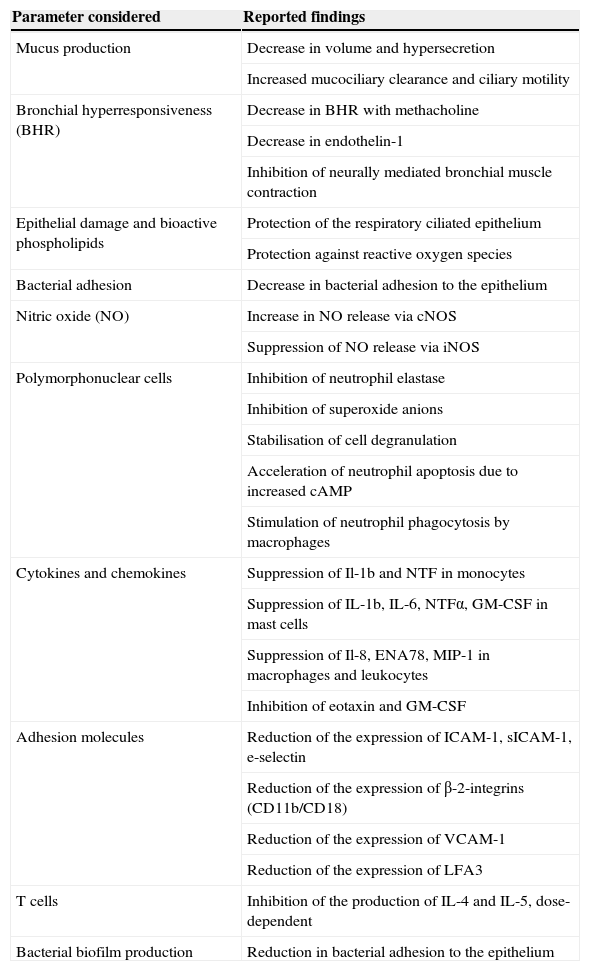
To sum up, there have been descriptions of interactions with elements of the inflammatory response mechanisms such as nitric oxide (NO)46,47, chemokines, transcription factors, endothelial remodelling and growth factors, bioactive phospholipids48, immune cells, etc. The scenario thus depicted is sufficiently suggestive to consider the possible use of these drugs in bronchopulmonary disorders presenting an inflammatory basis49 (Table 2).
Anti-inflammatory and immune modulating effects of the macrolides
| Parameter considered | Reported findings |
| Mucus production | Decrease in volume and hypersecretion |
| Increased mucociliary clearance and ciliary motility | |
| Bronchial hyperresponsiveness (BHR) | Decrease in BHR with methacholine |
| Decrease in endothelin-1 | |
| Inhibition of neurally mediated bronchial muscle contraction | |
| Epithelial damage and bioactive phospholipids | Protection of the respiratory ciliated epithelium |
| Protection against reactive oxygen species | |
| Bacterial adhesion | Decrease in bacterial adhesion to the epithelium |
| Nitric oxide (NO) | Increase in NO release via cNOS |
| Suppression of NO release via iNOS | |
| Polymorphonuclear cells | Inhibition of neutrophil elastase |
| Inhibition of superoxide anions | |
| Stabilisation of cell degranulation | |
| Acceleration of neutrophil apoptosis due to increased cAMP | |
| Stimulation of neutrophil phagocytosis by macrophages | |
| Cytokines and chemokines | Suppression of Il-1b and NTF in monocytes |
| Suppression of IL-1b, IL-6, NTFα, GM-CSF in mast cells | |
| Suppression of Il-8, ENA78, MIP-1 in macrophages and leukocytes | |
| Inhibition of eotaxin and GM-CSF | |
| Adhesion molecules | Reduction of the expression of ICAM-1, sICAM-1, e-selectin |
| Reduction of the expression of β-2-integrins (CD11b/CD18) | |
| Reduction of the expression of VCAM-1 | |
| Reduction of the expression of LFA3 | |
| T cells | Inhibition of the production of IL-4 and IL-5, dose-dependent |
| Bacterial biofilm production | Reduction in bacterial adhesion to the epithelium |
There is an important body of clinical, laboratory test and epidemiological evidence suggesting the implication of atypical bacterial species in the exacerbations of asthma and in development of the disease—although the precise contribution of these microorganisms to the development and maintenance of asthma remains unclear.50
On the other hand, the abundance of data on the anti-inflammatory effects of the macrolide antibiotics, based on both in vitro and in vivo studies, suggests that these substances exert actions additional to their strict antibacterial effects in patients with pulmonary inflammatory diseases. This dual activity, either synergically or separately, could explain the beneficial effects recorded in some controlled trials.
In a recent Cochrane review of the studies on macrolide treatment in asthmatic patients, a first version from the year 200351 identified only five studies meeting the screening criteria (i.e., randomised, double-blind and placebo-controlled studies involving at least four weeks of active treatment): Nelson 199352; Kamada 199353; Shoji 199954; Amayasu 200055; and Black 2001.56
A posterior version from the year 2005 identified 99 possible studies, of which a preliminary evaluation reduced the number of studies potentially eligible for inclusion in a systematic review to 25. In the end, only seven studies57 were seen to meet the aforementioned screening criteria. Specifically, two studies, published by Kraft in 200258 and Kostadima in 200459, were added to the five studies of the first version of the Cochrane review.
The 18 discarded studies were eliminated for one or more of the following reasons: the studies were uncontrolled, included patients other than asthmatic individuals, constituted in vitro studies, or the active treatment period lasted less than four weeks. A posterior version of the review corresponding to the year 2007 identified no further studies amenable to inclusion. The number of patients included in the first review totalled 397, to which 150 patients were added in the latter two studies included in the current version of the review: 55 from the Kraft study and 75 from the Kostadima study—thus representing a total of 507 patients analysed in the current metaanalysis.
The authors extracted the following primary conclusions from the review:
- •
There are no significant differences between the placebo group and the active treatment group in terms of the FEV1 or forced vital capacity (FVC) values.
- •
There are no significant differences in oral corticosteroid use between the two groups.
In turn, the following secondary conclusion was drawn:
A significant difference in symptoms reduction is observed in favour of the active treatment group (−1.25 standard deviation (SD) units, with 95% confidence interval −1.8–−0.7) (Amayasu 2000; Shoji 1999).
Amayasu in 2000 and Kostadima in 2004 reported an increase in the amount of methacholine required to achieve a 20% drop in FEV1 among asthmatic patients after treatment with clarithromycin. Due to the different provocation test methodologies used by the different groups, no metaanalysis of the joint results could be made.
Despite these findings, the authors concluded that there is some evidence in favour of the clinical usefulness of macrolides in the treatment of asthma. In this context, and in addition to the small number of patients analysed, it must be taken into account that the drugs used in each trial were different. In this context, troleandomycin, evaluated by Nelson and Kamada in 1993, showed no corticosteroid sparing effect and was suspended because of hepatic adverse effects. Roxithromycin (Shoji and Black) and clarithromycin (Amayasu, Kraft and Kostadima), corresponding to the new type of macrolides, have demonstrated anti-inflammatory effects in vitro, although their impact upon corticosteroid use has not been evaluated. There were no adverse effects of note, yet here again the limited number of patients studied does not allow the drawing of firm conclusions.
The results of a double-blind, randomised, placebo-controlled pilot study point in the same direction. Hahn et al.60 administered azithromycin 600mg/day for 3 days and then 600mg/week for 5 consecutive weeks. A total of 45 adults with stable asthma were included. Quality of life was assessed based on the Juniper Asthma Quality of Life Questionnaire (AQLQ), with evaluation of the asthma symptoms and medication changes from the start of the study until three months after the end of the study. In the patients with anti-C. pneumoniae IgA, the asthma symptoms improved significantly, and the use of rescue medication decreased −0.59 (−0.5, −1.6) (with 95% confidence interval) in the active treatment group versus the placebo group. No changes were recorded in the quality of life questionnaire.
Godfried61, in a double-blind, placebo-controlled study of 21 adults with corticosteroid-dependent asthma, administered clarithromycin 500mg twice a day for 6 weeks, and recorded an increase in FEV1 with a drop in nocturnal dyspnoea.
In the largest randomised trial published to date (278 patients), asthma exacerbations were treated with telithromycin 800mg/day for 10 days, or placebo. The active treatment group showed faster symptoms resolution and a significant increase in FEV1 versus baseline. This effect was lost after one month, however. The patients who benefited from treatment were mainly those with positive serology for acute M. pneumoniae or C. pneumoniae infection—thus pointing to a fundamentally antibacterial effect of this macrolide.62
Another multicentre, randomised double-blind, placebo-controlled and parallel group study in children between 6–17 years of age with persistent moderate to severe asthma evaluated the efficacy of azithromycin and montelukast versus placebo as inhaled corticosteroid sparing agents. The study was suspended prematurely because of a lack of efficacy of both drugs as corticosteroid sparing agents.63
Based on a recently developed experimental model in mice sensitised to ovoalbumin, involving the development of allergy after administration of the allergen64 the animals were treated with azithromycin, and a reduction in airway inflammation was observed as a result. The leukocyte count was seen to decrease in lung tissue and in bronchoalveolar lavage (BAL). The treatment moreover attenuated the expression of cytokines IL-5 and IL-13, and chemokines (CCL2, CCL3 and CCL4) in BAL. A decrease in mucosal cell metaplasia was observed. The results were similar regardless of whether the treatment was administered before provocation with the allergen, or afterwards.
Lastly, mention must be made of the increase in macrolide use among asthmatic patients registered in some countries. Kozyrskyj et al.65, in a study of the University of Manitoba (Canada) in paediatric patients, recorded an eight-fold increase in the use of these antibiotics between 1995 and 2001. In this sense, general practitioners prescribed these drugs twice as frequently as paediatricians. Previously, Pennie et al.66, in another Canadian study, had reported that 64% of all asthmatic children had received an antibiotic for respiratory infection, versus only 2% of the non-asthmatic children.
ConclusionsIt is necessary to determine the role played by the atypical bacteria M. pneumoniae and C. pneumoniae in the development, maintenance and exacerbation of asthma. It has even been speculated that these microorganisms could intervene in remodelling, i.e., the process ultimately responsible for the persistent obstruction seen in evolved asthma.
In this context, it seems clear that in patients with active infection caused by these bacteria, the response to treatment with macrolides is faster, more intense and with better results in terms of both the symptoms and bronchial hyperresponsiveness—though the response does not seem to persist over time.
It seems clear that certain members of the macrolide antibiotic family possess anti-inflammatory and immune modulating effects that extend beyond their antibacterial activity. Randomised, controlled clinical trials involving larger patient samples are therefore needed to confirm whether these actions are of clinical relevance in application to asthma.
On the other hand, the macrolide antibiotics have a long half-life, with a prolonged elimination interval, which appears to favour the development of resistances which persist over the long term, as in the case of azithromycin. Would the risk/benefit ratio of sustained low-dose macrolide use therefore be justified, considering the risk of selecting resistant strains?
A number of questions must be answered before these drugs can be recommended in application to asthmatic patients:
- •
Is the use of macrolides in application to asthma advisable?
- •
In which patients should they be used?
- •
Which drug or drugs would be most appropriate?
- •
What would the recommended dose be, and for how long should treatment be administered?
- •
What adverse effects can be expected?