Pollens represent a rich source of proteins that are also potential elicitors of IgE-mediated pollen allergy. Sensitisation to panallergens could play an important role in diagnosis and specific immunotherapy, because these molecules are present in different plant pollens and plant foods and have marked structural similarity in different species. Profilins are one of the most common panallergens to be studied because they are responsible for a large number of sensitisations and are clearly related to cross-reactivity and co-sensitisation. This study aimed to isolate and characterise a new allergen of Amaranthus palmeri pollen and to determine its allergenicity.
MethodsA. palmeri pollen profilin was purified using poly-l-proline-Sepharose affinity chromatography followed by anion exchanger chromatography. Identification of purified protein was carried out by mass spectrometry. Specific IgE was estimated in sera of patients with positive skin prick test to A. palmeri pollen extract, by enzyme-linked immunosorbent assay (ELISA).
Principal findingsPurified protein appeared as a single band at 14kDa in SDS-PAGE gel. Mass spectrometric analysis of the gel band identified two highly conserved peptides corresponding to allergenic profilins from pollen of other plants. Sera from about 60% of allergic patients have IgE that recognises the purified A. palmeri protein.
ConclusionA 14kDa protein of A. palmeri pollen was purified and identified as allergenic profilin, which was recognised by sera from pollen allergic patients.
Allergy represents the most prevalent human disorder, affecting almost 30% of the population in developed and developing countries.1 Allergic diseases include rhinoconjunctivitis, allergic asthma, and allergic dermatitis.1,2 Allergic tissue inflammation can be mitigated by anti-inflammatory drugs and immunosuppressive agents, however, only allergen-specific immunotherapy is considered as an effective and efficient treatment, with long-lasting clinical effects and can prevent the progression of mild forms of allergy to severe manifestations.3 Recently, genetically engineered hypoallergenic derivatives have been incorporated to desensitisation immunotherapy.4 This strategy requires detailed molecular and immunological studies to identify and design hypoallergenic molecules that can be used in allergen-specific immunotherapy.3
Recent reports indicate that around half of allergic people present reactions to pollen allergens from different plants.5 Furthermore, pollen-allergic patients frequently exhibit allergic symptoms after the ingestion of several kinds of plant-derived foods. This might be simply attributed to poly-sensitisation to different allergenic plants.6 Another explanation for this phenomenon is the concept of IgE cross-reactivity between functional and structural related allergens, which are known as panallergens.
Panallergens are evolutionarily conserved, ubiquitous components of several complex sources of allergens, which usually act as minor allergens. However, their presence has important clinical implications in establishing the phenomenon of food-pollen cross-reactivity, in the interpretation of diagnostic tests and in the preparation of immunotherapy extracts.
One of the most studied pollen and plant-derived food panallergens is profilin, a well-known ubiquitous cytoskeleton protein that is highly conserved among eukaryotic cells. Binding sites and interacting motifs for different substrates have been characterised in many profilins, including plant proteins. In addition, secondary and tertiary structures of all profilins so far elucidated are amazingly similar. Profilins are involved in different molecular and cellular processes through binding to actin monomers,7 actin-related proteins (ARPs), poly-l-proline (pLp) stretches,8 and phosphatidylinositol lipids.9 Profilin was recognised as an allergen for the first time in birch pollen.10 Then, it was described as a relevant allergen in plant-derived foods, principally celery, carrot, peach, pear, apple, potato, tomato and pumpkin seed, which suggests that profilins could be responsible, at least in part, for cross-reactions between different allergens sources.5,11
Different clinical studies show that Amaranthus palmeri pollen is an important allergen source in the USA, Thailand and Mexico.12–14 However, little is known about the clinically relevant allergens of this pollen.
To gain insights into the allergens of A. palmeri pollen, in this work we performed the isolation and purification of a profilin from pollen grains and evaluated its allergenic proprieties. Our results allowed the identification of four profilin isoforms in A. palmeri pollen and demonstrated their recognition by IgE from sera of A. palmeri-allergic people.
Materials and methodsThe study was approved by the Ethical Committee of the National School of Medicine and Homeopathy of National Polytechnic Institute, Mexico and was carried out in compliance with the guidelines of the Helsinki Declaration of 1975. Participants gave their written informed consent.
Pollen extract preparationPollen from A. palmeri (purity higher than 95%), was purchased by Antígenos Allergomex Distribuidora S.A. de C.V., Pollen was defatted by repeated changes of ethanol-acetone and diethyl ether. Defatted pollen (1g) was suspended in 10mL of 50mM carbonate buffer (50mM Na2CO3-NaHCO3, 5mM EDTA and 0.5mM PMSF; pH 8.0) and shaken for 16h at 4°C. Pollen grains were separated by centrifugation at 10,000×g for 15min, the supernatant was filtered through a 0.22μm pore size PVDF membrane (Millipore) and dialysed against 50mM carbonate buffer pH 8.0. Total proteins in aqueous pollen extract were quantified using the BCA Protein Assay kit (Pierce, USA) and protein integrity was verified by SDS-PAGE.
Chromatographic isolation of profilin isoformsProfilin isoforms were purified by affinity chromatography using a poly-l-proline-Sepharose support. Briefly, protein extract was applied to a home-made pLp-Sepharose support at a flow rate of 0.5mL/min. Unbound proteins were washed away with 50mM carbonate buffer (20 column volumes), and 1M guanidine hydrochloride (two volumes) in ultrapure water. Then, retained profilins were eluted with 4M guanidine hydrochloride and dialysed against 50mM carbonate buffer, pH 8.0. Finally, eluted proteins were fractionated through an anion exchange column (Mono Q™ GE Healthcare) coupled to a HPLC (1220 Infinity LC, Agilent Technologies) equilibrated with 50mM phosphate buffer, pH 7.4. Retained proteins were eluted with the same buffer containing 1M NaCl. Each fraction was analysed by 16% SDS-PAGE and Coomassie Blue staining to evaluate profilin homogeneity. Protein concentration was determined by the bicinchoninic acid method.
Immunodetection of profilinA. palmeri pollen extract and profilin obtained from affinity-chromatography were separated by 16% SDS-PAGE and electro-transferred onto polyvinylidene difluoride (PVDF) membranes (Immobilon-P, Millipore) using standard methods. Membranes were completely dried to block unoccupied sites and prevent nonspecific binding of antibodies. Then, membranes were incubated at room temperature for two hours with a monoclonal antibody against Arabidopsis thaliana profilin (Sigma–Aldrich®) (1:3000 dilution). After washing with PBS-Tween 0.05%, membranes were incubated for 1h at room temperature with an anti-mouse-IgG-HRP antibody (BioLegend®) (1:5000 dilution). Finally, bound antibodies were detected using 3,3-diaminobenzidine (Sigma–Aldrich®) as substrate.
Profilin-specific monoclonal antibody bindingThe main Mono Q fractions obtained from affinity chromatography fraction were analysed by ELISA. Briefly, immunoplates (Corning Inc.) were coated with 10μg of protein per well diluted in coating buffer (0.05M carbonate bicarbonate buffer pH 9.6). Plates were incubated overnight at 4°C, washed with PBS-Tween 0.25% and blocked with 5% blocking agent (BLOT-QuickBlocker®, Millipore) in PBS at 25°C for 2h. After washing with PBS-Tween solution, 100μl of a monoclonal antibody against A. thaliana profilin (1:5000 dilution) were added in each well and incubated for one hour at room temperature. Plates were washed again and incubated for 1h at room temperature with an anti-mouse-IgG-HRP antibody (1:5000 dilution). Chromogenic substrate was prepared by adding 50μl of a tetramethylbenzidine (TMB) solution (6mg/ml in dimethyl sulfoxide) and 1.5μl of 3% H2O2 to 2.5ml of 0.1M sodium acetate (pH 5.5) solution. One hundred microlitres of the chromogenic substrate were added to each well. After 15min of incubation in the dark, colour development was stopped by addition of 100μl of 2N H2SO4. Optical density was read at 450nm using an ELISA plate reader (Stat Fax 303+, Awareness Technology, Inc.).
Profilin identification by mass spectrometryMono Q column fractions that were detected as positive in the ELISA experiment described above, were first analysed by automated Edman degradation on a gas-phase protein sequencer (LF 3000, Beckman Instruments, Irvine, CA, USA). Because this experiment failed to identify protein (see below), proteins were then identified by mass spectrometry. For this, other aliquots of Mono Q fractions were submitted to 16% SDS-PAGE and Coomassie blue staining. Bands corresponding to about 14kDa, which is the expected molecular weight for profilin, were manually excised from polyacrylamide gel with a sterile scalpel. Gel pieces were washed twice with 50% (v/v) acetonitrile in 25mM ammonium bicarbonate (pH 8.5) for 15min to remove Coomassie dye. After dehydration with 100% (v/v) acetonitrile for 10min at room temperature, gel pieces were vacuum-dried and rehydrated with sequencing-grade modified trypsin (Promega, Madison, WI, USA) in 25mM ammonium bicarbonate (pH 8.5) at 37°C overnight. Then, in-gel tryptic digested samples were separated by Capillary HPLC and analysed by Electrospray tandem mass spectrometry (ESI-MS/MS) using a 3200 Q TRAP hybrid tandem mass spectrometer (Applied Biosystems/MDS Sciex, Concord, ON, Canada). Peptide mass and sequence were established from MS data and MS/MS fragmentation patterns of the peptides. Protein identification was performed by searching a non-redundant protein sequence database (NCBI) restricted to green plants using the Mascot search engine (http://www.matrixscience.com).
Detection of IgE against A. palmeri profilin by ELISAThe presence of specific-IgE that recognise A. palmeri profilins was evaluated by ELISA in serum of 15 allergic people with pollinosis, manifested by disease history and positive skin prick test (SPT) to A. palmeri pollen extract and other pollens. Five subjects with negative SPT responses were used as negative controls. Briefly, immunoplates were coated with purified profilin as described above. Wells were then incubated with patients’ serum (1:10 in PBS-Tween 0.05%) for 3h at 25°C and 15h at 4°C. After washing, biotinylated anti-human IgE (Zymed®) was added to each well (1:4000) and incubated for 2h at 25°C. The immunoplate was washed and incubated for 2h with streptavidin-horseradish peroxidase conjugate (Zymed®) at room temperature. Enzymatic reaction was detected using tetramethylbenzidine as substrate; the reaction was stopped with 2N H2SO4 after 15min incubation at room temperature and optical density was read at 450nm (OD450). A sample was considered positive when the OD450 value was higher than the mean value obtained for SPT negative people plus three standard deviations.
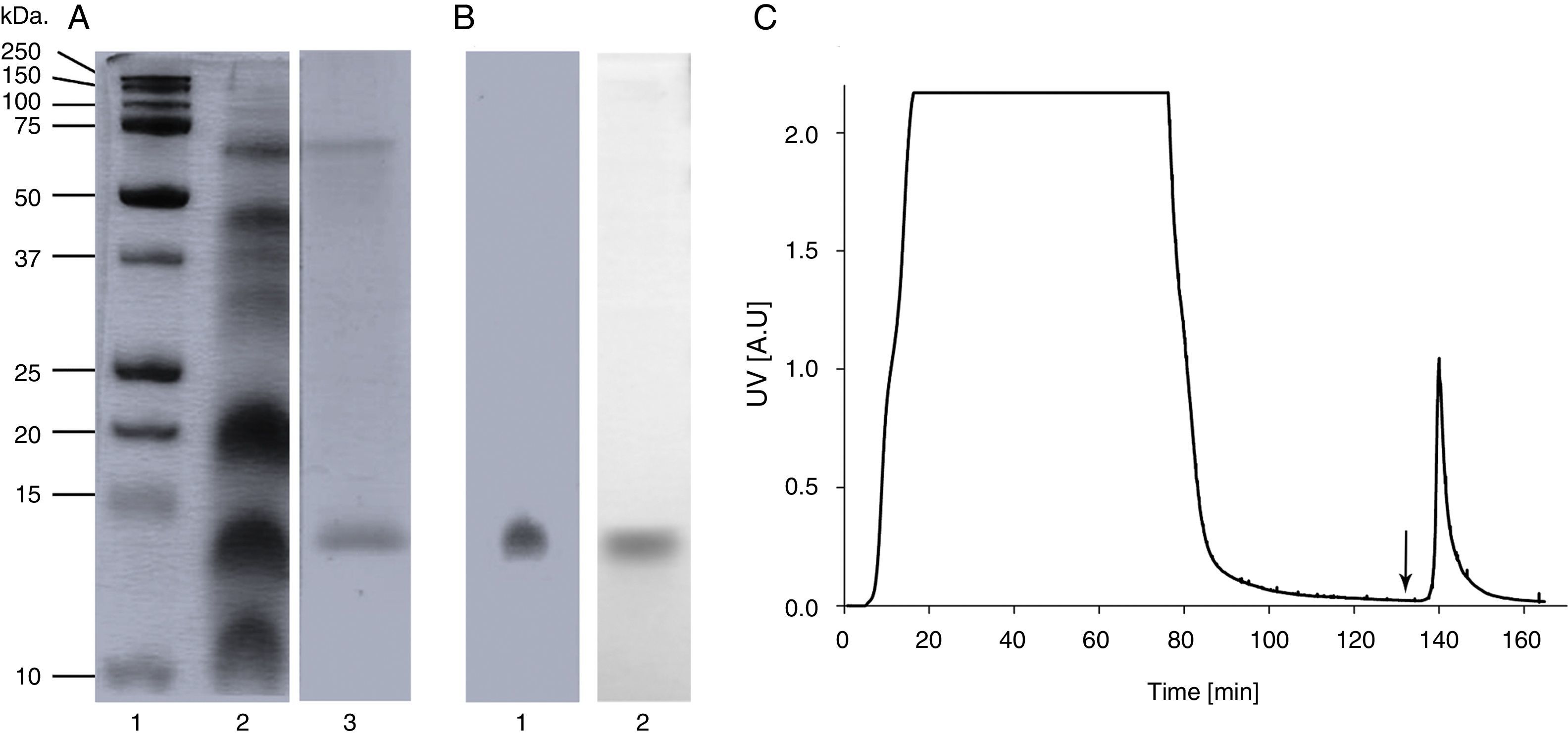
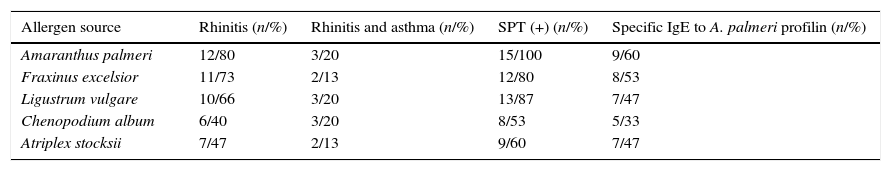
ResultsA. palmeri pollen has a protein fraction that was recognised by an anti-profilin monoclonal antibodySDS-PAGE analysis of A. palmeri pollen extract revealed the presence of several proteins with molecular masses ranging from 12 to 75kDa (Fig. 1A). Interestingly, profilin-specific monoclonal antibody immunodetected a unique band with a molecular mass of 14kDa, which probably corresponds to A. palmeri profilin (Fig. 1A). Because profilins have conserved binding sites for poly-l-proline (pLp), we purified this allergen from A. palmeri pollen by affinity chromatography using a pLp-Sheparose column. Proteins with pLp affinity were obtained in a single elution fraction (Fig. 1C) with a yield of 150μg per gram of A. palmeri pollen processed. SDS-PAGE analysis confirmed the presence of a predominant band of about 14kDa, which corresponds to the expected molecular weight for profilin (Fig. 1B). Notably, this band was immunodetected by a monoclonal antibody against profilin from A. thaliana, which strongly suggests the presence of homologous proteins in this fraction (Fig. 1B).
Purification of profilin of A. palmeri pollen by Affinity chromatography. (A) SDS-PAGE of pollen extracts. Total and purified proteins were separated on 15% acrylamide gel and stained by colloidal Coomassie blue. Lane 1, molecular weight markers (kDa); lane 2, total protein extract; lane 3, purified proteins. (B) Immunodetection of profilin. Total and purified proteins were immunodetected by monoclonal antibody against profilin from A. thaliana. Lane 1, total protein extract; lane 2, purified proteins. (C) Elution profile of retained proteins from p-L-p-Sheparose column (arrow indicate addition of 4M of guanidine–HCl).
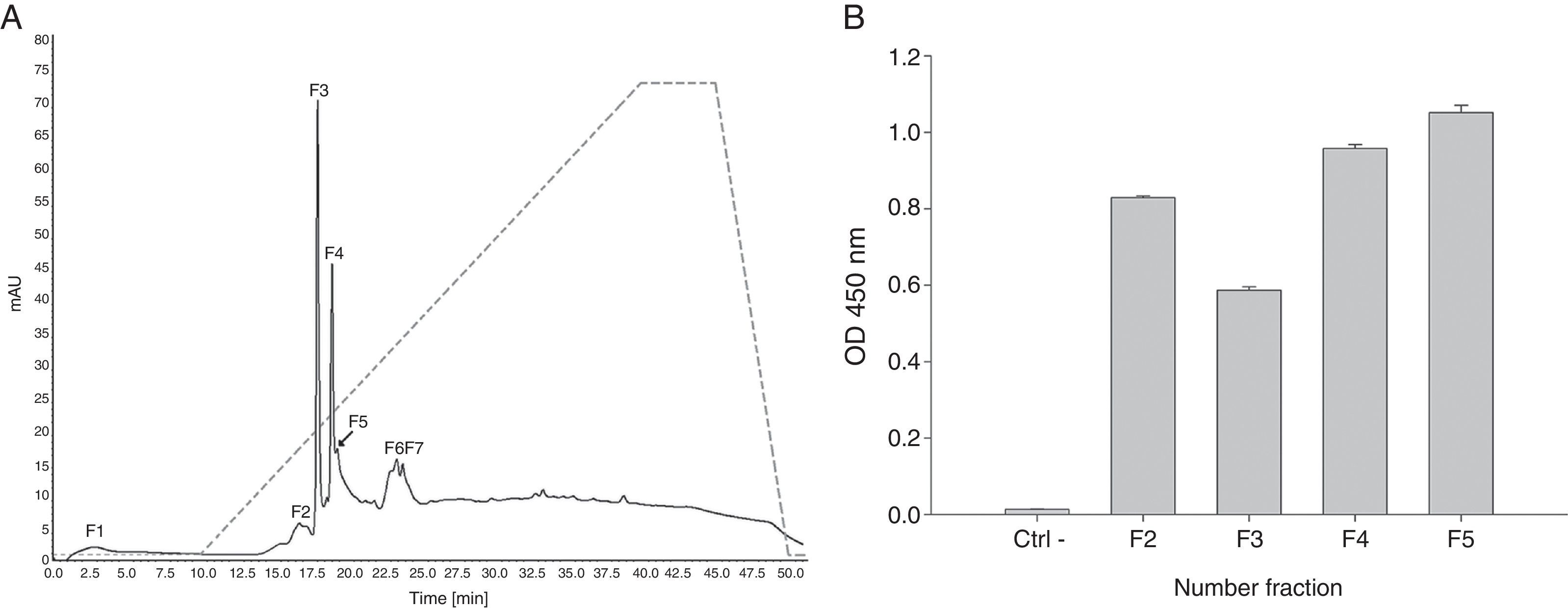
Proteins retained in pLp-Sepharose column were then submitted to anionic-exchange chromatography using a Mono Q column. Results evidenced the separation of seven main fractions (Fig. 2A). Notably, fractions F2–F5 were recognised by a monoclonal antibody against profilin from A. thaliana pollen in ELISA (Fig. 2B). Altogether, these results indicate the presence of at least four profilin isoforms in A. palmeri pollen extract.
Anion-exchange chromatography on Mono Q and ELISA assay. (A) Ion-exchange chromatography of profilin retained from affinity column. Solid line represents A280; segmented line represents salt gradient up to 1M NaCl. Major fractions are labelled F1–F7. (B) Fractions recognised by a monoclonal A. thaliana profilin antibody in ELISA. Antibody against A. thaliana profilin binding with no protein in wells was used as negative control (Ctrl−).
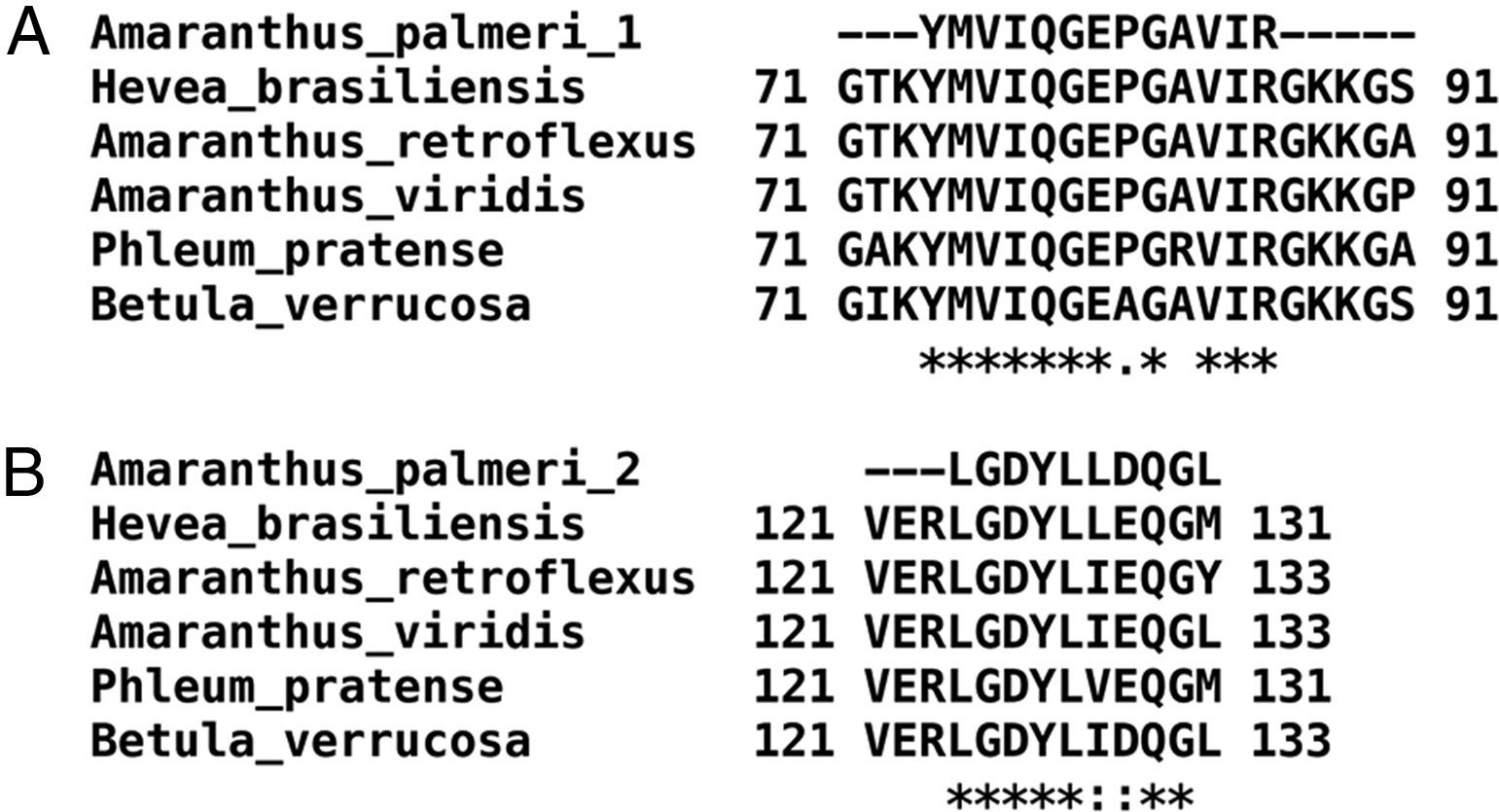
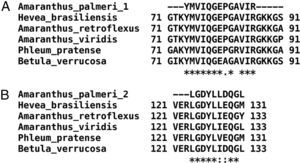
Only fractions F4 and F5 were submitted to mass spectrometry analysis, because fractions F2 and F3 were insoluble proteins. In both fractions, mass spectrometry analysis identified two peptides that correspond to the sequence of profilin from other plants. Particularly, peptide 1 (YMVIQGEPGAVIR) totally matches with amino acids 72–84 in profilin from Hevea brasiliensis, while peptide 2 (LGDYLLDQGL) corresponds to the 122–131 amino acids region of the same protein. Moreover, regions corresponding to both peptides are highly conserved among allergenic profilins obtained from the allergome database (http://www.allergome.org/) and aligned with clustal-W software (Fig. 3).
Comparison of identified A. palmeri peptide amino acid sequences with profilins from other allergenic sources. (A) Peptide 1 (internal peptide) and (B) peptide 2 (C-terminal peptide). Sequence identity (*) and amino acid changes with conserved physicochemical properties (:) of peptides identified in A. palmeri pollen profilin were identified through compared with sequences of profilins from highly allergenic sources from H. brasiliensis (CAA75312), A. retroflexus (ACP43298), A. viridis (ABW37744), P. pratense (Y09457) and B. verrucosa (P25816).
Patients’ characteristics and sensitisation profiles are summarised in Table 1. Respiratory symptoms of rhinitis were reported in 12 of 15 patients (80%) and rhinitis and asthma symptoms were reported in three of 15 (20%) patients. No food allergy symptoms were reported in any patient. High prevalence of sensitisation to Fraxinus excelsior, Ligustrum vulgare, Chenopodium album and Atriplex stocksii pollen were reported in patients with positive SPT to A. palmeri pollen.
Sensitisation profiles as estimated by skin prick testing or specific immunoglobulin E determination.
| Allergen source | Rhinitis (n/%) | Rhinitis and asthma (n/%) | SPT (+) (n/%) | Specific IgE to A. palmeri profilin (n/%) |
|---|---|---|---|---|
| Amaranthus palmeri | 12/80 | 3/20 | 15/100 | 9/60 |
| Fraxinus excelsior | 11/73 | 2/13 | 12/80 | 8/53 |
| Ligustrum vulgare | 10/66 | 3/20 | 13/87 | 7/47 |
| Chenopodium album | 6/40 | 3/20 | 8/53 | 5/33 |
| Atriplex stocksii | 7/47 | 2/13 | 9/60 | 7/47 |
Abbreviations: SPT (+), positive skin prick test.
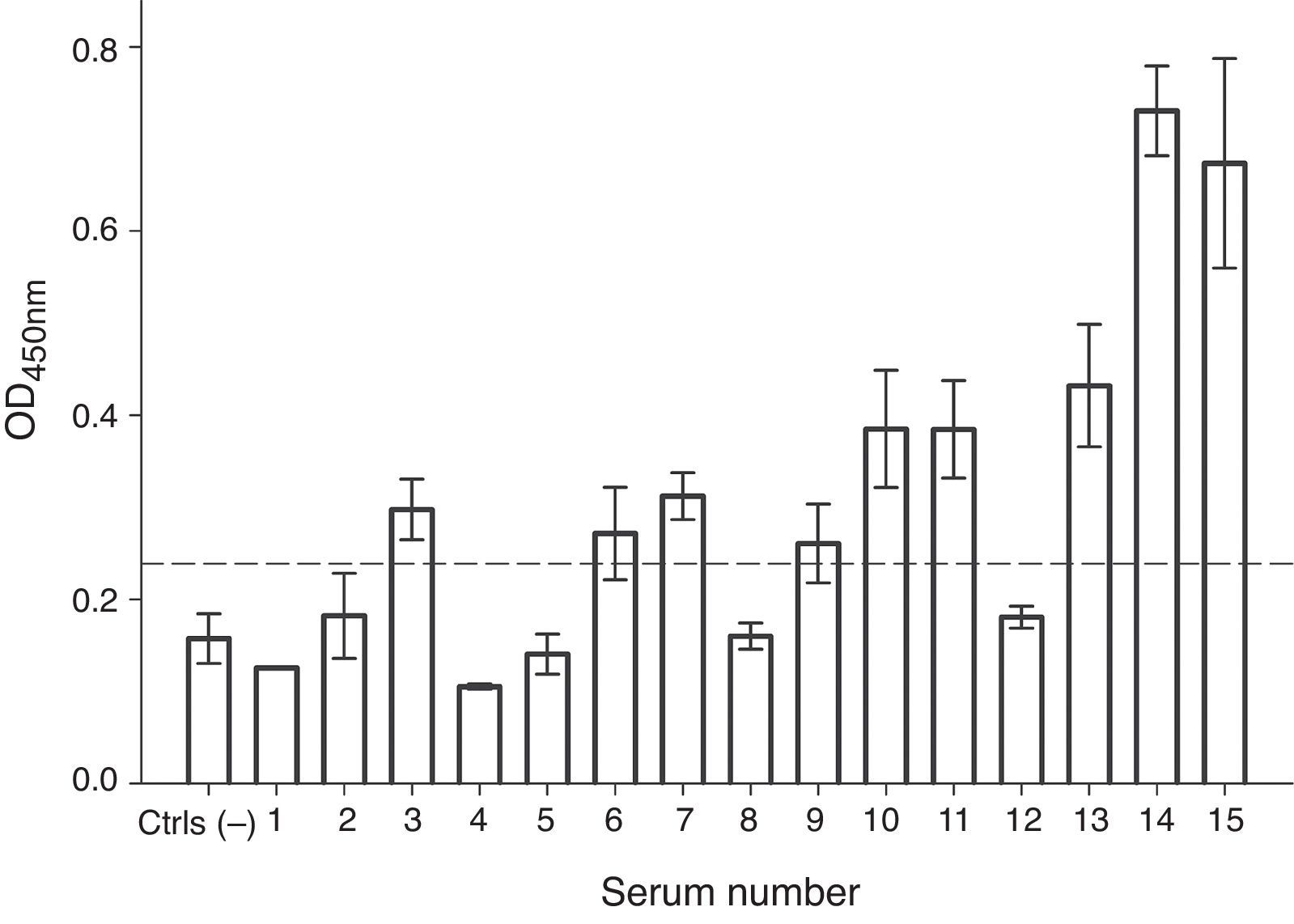
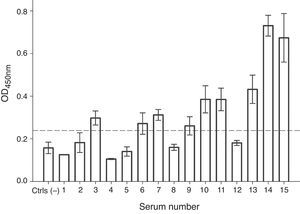
In order to evaluate the allergenicity of A. palmeri pollen profilin, we analysed serum from 15 individual patients’ with positive SPT to A. palmeri pollen extract by ELISA, using the profilins contained in the fraction obtained from affinity chromatography as antigen. Result showed that 9 out of 15 sera contain specific IgE antibodies that recognised profilins coated in wells, representing 60% of the sera tested (Fig. 4).
IgE recognition of profilin by individual sera from patients hypersensitive to A. palmeri pollen. ELISA assay responses for sera of 15 patients. A pool of five non-allergic subject serum was used as controls (Ctrls−). A sample was considered positive when the OD450 value was higher than the mean value obtained for skin prick test negative people plus three standard deviations. The error bars indicate the standard deviation between ELISA replicates.
Because of the high amino acid sequence identity between profilins from different organisms and consequently their comparable immunogenicity, profilins have been described as pan-allergens of various plant species.15 In this study, the identification of profilin from A. palmeri pollen and the presence of profilin-specific IgE antibodies in serum of patients is reported. In A. palmeri, at least four profilin isoforms are expressed in pollen, as demonstrated by anion-exchange chromatography and ELISA using a monoclonal antibody against A. thaliana pollen profilin. Similarly, several isoforms have been reported for allergenic profilin from pollen of Ambrosia artemisiifolia (isoforms Amb a 8.01 and Amb a 8.02),16Amaranthus viridis (Ama v 2.0101 and Ama v 2.0201),17Phleum pratense (Phl p 12.0101, Phl p 12.0102, Phl p 12.0103),18Salsola kali (Sal k 4.0101, Sal k 4.0201, Sal k 4.0301),19 among other pollens. Purification yield of profilin by affinity chromatography was 150μg/g of pollen, slightly higher than previously reported for other pollens using similar purification protocols, such as sunflower, olive and lambsquarters, which showed yields between 30 and 100μg/g of pollen.20–23 Moreover, purity of profilin after anion-exchange chromatography was more than 95% according to SDS-PAGE and colloidal Coomassie Blue staining.
Fractions (F4 and F5) from anion-exchange chromatography containing profilin isoforms were initially subjected to automated Edman sequencing; however assays failed to release a NH2-terminal residue from these fractions (data not shown). These results suggested the presence of a possible post-translational modification of profilin from A. palmeri pollen. NH2-terminal blockage typically occurs when the α-amino groups are acylated or when the N-terminal residue is a pyrrolidone carboxylic acid formed by cyclisation of glutamine.24 Proteins with serine and alanine termini are the most frequently acetylated, plant profilins display a very high degree of amino acid sequence homology in N-terminal segment (residues 1–15).23,25,26 Importantly, serine 2 is present in all reported sequences of allergenic profilins from pollen, which reinforced the idea that a post-translational modification is present in A. palmeri pollen profilin isoforms, most likely, an acetylation of the N-terminal serine. Allergenic profilins with blocked N-terminal amino acid have also been reported in melon26 and olive pollen.27 This N-terminal modification has also been identified in other plant allergens, such as lipid transfer protein from peach28 and isoflavone reductase from birch pollen.29 Because it was not possible to obtain the N-terminal sequence of amino acids from isolated profilin isoforms through Edman degradation method, we performed their identification by mass spectrometry. The detection of two peptides that showed highly conserved amino acid sequence found in profilins from allergenic pollen of a large number of plants, confirmed the identification of profilin isoforms in A. palmeri. Identified peptides are contained in a region that has been previously reported as part of a major IgE binding-site in profilins from H. brasiliensis, Betula pendula, and A. thaliana.30 Preliminary results obtained by our group show that profilin isoforms identified in this work have a molecular mass of 13,950Da for isoform present in F4 and of 13,958Da for profilin in F5, as judged by MALDI-TOF mass spectrometry analysis (data no shown), which is consistent with the expected molecular mass for these proteins. This result reinforces earlier evidence that identified allergen belongs to profilin family.
On the other hand, ELISA analysis revealed that 60% of serum from patients with positive SPT to A. palmeri pollen showed specific IgE to pooled profilin isoforms. Similar sensitisation rates were observed in patients with positive SPT to F. excelsior pollen (53%). Slightly lower rates of sensitisation were observed in patients with positive SPT to L. vulgare and A. stocksii pollen (47%), suggesting a possible role of A. plameri profilin in cross-reactivity among these pollens. Interestingly, patients with positive SPT to C. album pollen, a plant of Amaranthaceae/Chenopodiaceae family, only 33% showed IgE to A. palmeri profilin. In contrary to our finding, previous reports showed sensitisation rates of 55% to profilin from C. album pollen in Spanish patients.23 It is to note that profilin has been found to be a minor but important allergen in pollen from birch (20%), timothy (20%),15 mugwort (20–36%),15,31 olive (24–47%)27,32 and sunflower (30%).33 Nonetheless, it has been found to be a major allergen in other pollens, such as annual mercury (51%),34 saffron (53%),35 lambsquarters (55%),23 date palm (56–64%),36 and soybean (69%).37 Similar observations have been reported for profilin of some plant food extract such as muskmelon (71–100%),26,38 orange (78–87%)39 or watermelon, with a rate of profilin-sensitised patients of 56%.40
In conclusion, we have demonstrated that profilin from A. palmeri pollen is an important allergen for Mexican allergic subjects, which suggests that it can be considered as a panallergen allergen in this allergenic source. Further studies are required to determine whether profilin isoforms represent interesting candidates to be included in desensitisation protocols for people allergic to A. palmeri pollen.
Conflict of interestThe authors have no conflict of interest to declare.
Ethical disclosuresConfidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Protection of human subjects and animals in researchThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
We thank the Instituto Politécnico Nacional (grant SIP-2010-0232), SECITI-DF and CONACyT (grants 54626 and 106839) for support. César Manuel Landa Pineda was supported with fellowships from CONACyT.