Type 1 diabetes mellitus (T1DM) may be associated with allergy. It was previously reported that >20% of children with T1DM had allergic rhinitis (AR), but none was asthmatic. This finding was surprising as allergic rhinitis is frequently associated with asthma and asthma prevalence is about 10% of the general paediatric population. Thus, it was hypothesized that T1DM could protect from asthma.
ObjectivesThe aim of this preliminary study was to evaluate the pulmonary function and the response to bronchodilation testing in children, suffering from T1DM with associated AR, comparing them with a control group of children with AR alone.
MethodsTwenty children with T1DM and AR were compared with 59 children with AR alone; spirometry and bronchodilation testing were performed in all patients.
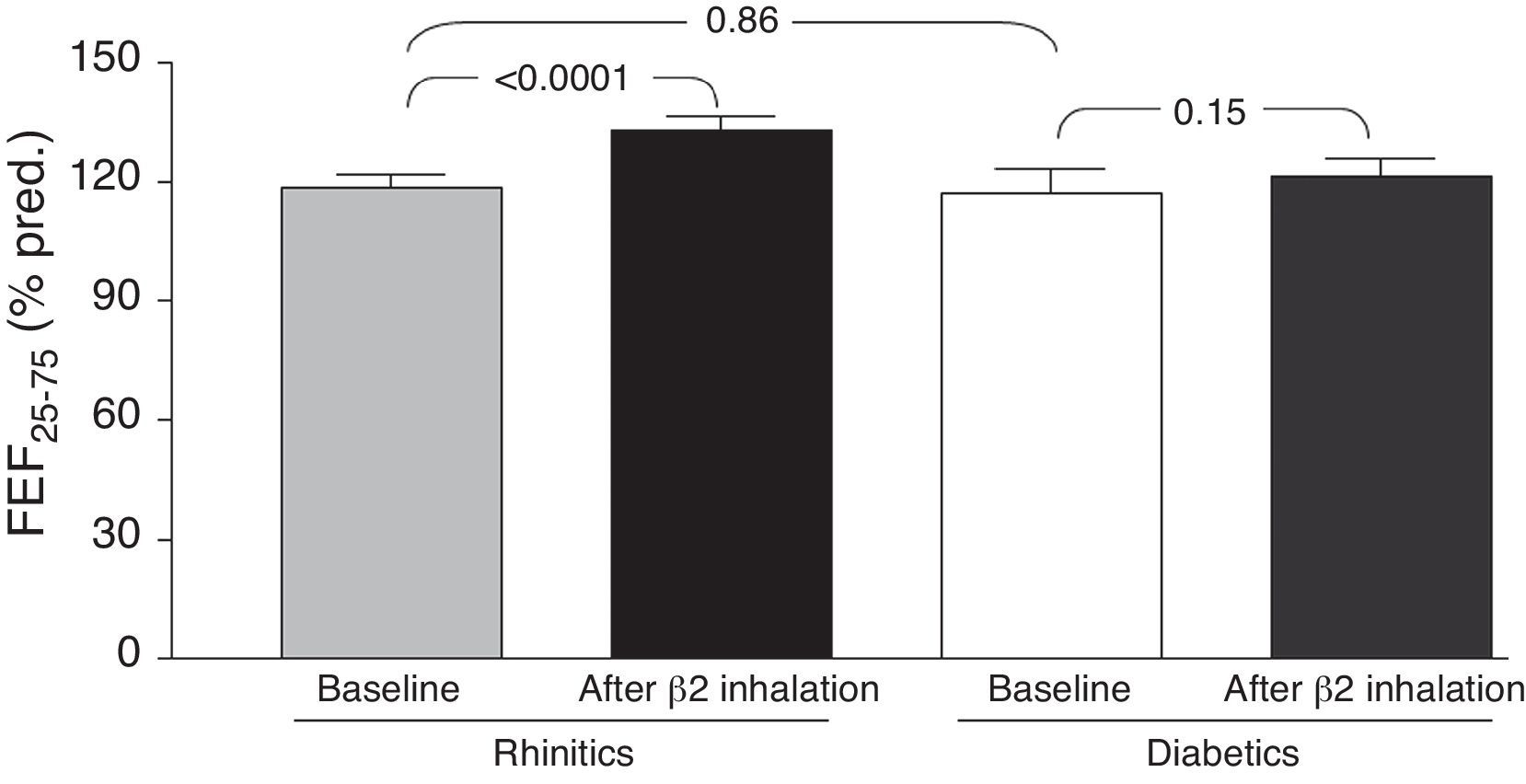
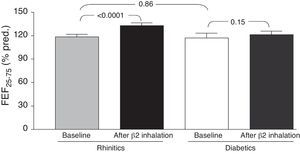
ResultsThere were no statistically significant differences in both “at baseline” and after bronchodilation testing about FVC, FEV1, and FEF25–75 values. However, changes in “post-bronchodilator” values of FEF25–75 (ΔFEF25–75) were significantly higher in children with AR alone than in children with T1DM and AR (p=0.04).
ConclusionsThis preliminary study could sustain the hypothesis that T1DM in children suffering also from AR might exert a protective effect of preventing the possible evolution in asthma.
Asthma and Type 1 diabetes mellitus (T1DM) are complex disorders, sustained by an immune dysregulation.1 T1DM is commonly considered a Th1-mediated autoimmune disorder, whereas asthma, specifically when associated to allergic sensitisation,2 is typically characterised by a Th2-dependent inflammation.3 However, the paradigm based on the assumption that Th1 and Th2 diseases should be mutually exclusive has to be substantially revised considering not only the relevant role of T regulatory cells (Tregs) played in both types of disorders4 but also the epidemiological data showing a frequent association between them.5 Indeed, T1DM does not seem to play a down-regulating role on the development of allergic sensitisation, but might lower the frequency or the severity of its clinical manifestations at respiratory level. In this regard, we previously reported that in a cohort of 112 T1DM teenagers nobody had current asthma even though 49 of them were sensitised to aeroallergens and 25 had allergic rhinitis.6 This finding was surprising for two reasons: (i) asthma affects about 10% of the general paediatric population, and (ii) allergic rhinitis is frequently associated with asthma. Therefore, the absence of asthma in all these T1DM children could support the idea that diabetes might protect from asthma. To further analyse this hypothesis, a preliminary study was designed to evaluate the pulmonary function and the response to bronchodilation testing in 20 consecutive children, suffering from T1DM with associated allergic rhinitis, comparing them with a control group of 59 consecutive children with allergic rhinitis alone.
Materials and methodsStudy populationA total of 20 children (11 males, median age 14.6 years) consecutively referred as outpatients to the Regional Childhood Diabetes Centre of the G. Gaslini Institute for T1DM management completed the study. The inclusion criteria were: diagnosis of both T1DM and allergic rhinitis. The exclusion criteria were: previous or current allergen-specific immunotherapy, and use of drugs that could interfere with the study. A control group of 59 consecutive children (31 males, median age 10.3 years) referring to the Allergy Centre of the same institution for allergic rhinitis management were enrolled. The inclusion criterion was the diagnosis of allergic rhinitis. The exclusion criteria were the same as for the study group.
The Institutional Ethical Committee of the G. Gaslini Institute approved the protocol. Signed informed parental consent and the child's assent (if the child was ≥10 years old) were obtained. Diabetes diagnosis was based on standardised and validated criteria, such as: (a) positive pancreatic β-cells autoantibodies; (b) signs and symptoms of diabetes mellitus; (c) insulin-dependence.7,8 Allergic rhinitis diagnosis was performed using validated criteria, such as: (a) presence of typical symptoms, (b) sensitisation to airborne allergens, and (c) symptom occurrence after exposure to the sensitising allergens.9
Data collectionInformation on demography, metabolic parameters, and presence of respiratory disorders (such as allergic rhinitis and asthma) was collected at the time of the survey. A questionnaire, a slightly modified version of International Study of Asthma and Allergies in Childhood (ISAAC),10 was used to investigate the frequency of asthma and allergic rhinitis. The questionnaire was administered to parents of all children enrolled in the study and the demographic and clinical data were collected by the physicians involved in the study. Information on current rhinitis symptoms (itchy and/or runny and/or blocked nose) and asthma-related symptoms (dyspnoea, wheezing, recurrent dry cough or exercise-related symptoms) was collected.
Skin prick testSkin prick tests were performed according to the methodology and criteria proposed by the European Academy of Allergy and Clinical Immunology (EAACI).11 The test uses a panel of allergens extracts, histamine solution (10mg/ml) as positive control and glycerol buffer diluents of allergenic preparations as negative control. Wheals at least 3mm×3mm greater than wheals at the site of negative control are regarded as positive.
Lung function measurementForced vital capacity (FVC), forced expiratory volume in 1s (FEV1), and forced expiratory flows at 25–75% of vital capacity (FEF25–75%) were measured by spirometry (Med Graphics, Pulmonary Function System 1070 series 2, Med Graphics Corp., St. Paul, MN), according to the guidelines provided by the American Thoracic Society.12 All the children were able to obtain at least three technically acceptable breathing manoeuvres with the spirometer. After the “at baseline” evaluation, spirometry was repeated 15min after two inhalations of a salbutamol metered dose inhaler (100mg/actuation) via a spacer device, and post-bronchodilation FVC, FEV1, FEF25–75, and FEV1/FVC ratio were recorded. Three forced expiratory manoeuvres were obtained, and the best values were retained. The results were expressed as percentage of the predicted and compared with reference values obtained from a well-defined population, identified by the American Thoracic Society, of healthy subjects comparable for gender, height, weight and then expressed as a percentage.13
Statistical methodsDescriptive statistics were firstly performed and qualitative data were reported in terms of absolute frequencies and percentages; means and standard deviations (SD), or medians and lower and upper quartiles (LQ-UQ) were reported for quantitative variables (age, BMI, pulmonary function test parameters, etc.) when appropriate. Comparison of qualitative data between groups was made by the chi-square test or by the Fisher's Exact test whenever expected frequencies were less than five. All tests were two sided and a P value less than 0.05 was considered statistically significant. The statistical package used was the “Statistica release 6” (StatSoft Corp., Tulsa, OK, U.S.A.) for all the analyses.
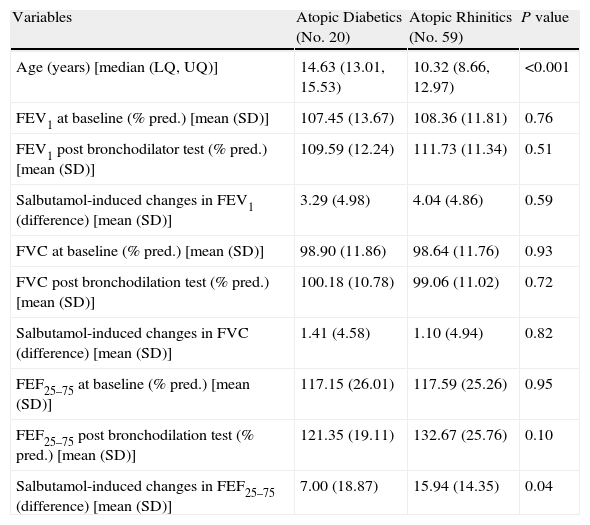
ResultsThe median age (with lower and upper quartiles) of the whole study population was 9.0 (12.2, 13.8). The Table shows the demographic and clinical characteristics of studied children (Table 1).
– Demographic and clinical characteristics in atopic diabetics and in atopic rhintics.
| Variables | Atopic Diabetics (No. 20) | Atopic Rhinitics (No. 59) | P value |
| Age (years) [median (LQ, UQ)] | 14.63 (13.01, 15.53) | 10.32 (8.66, 12.97) | <0.001 |
| FEV1 at baseline (% pred.) [mean (SD)] | 107.45 (13.67) | 108.36 (11.81) | 0.76 |
| FEV1 post bronchodilator test (% pred.) [mean (SD)] | 109.59 (12.24) | 111.73 (11.34) | 0.51 |
| Salbutamol-induced changes in FEV1 (difference) [mean (SD)] | 3.29 (4.98) | 4.04 (4.86) | 0.59 |
| FVC at baseline (% pred.) [mean (SD)] | 98.90 (11.86) | 98.64 (11.76) | 0.93 |
| FVC post bronchodilation test (% pred.) [mean (SD)] | 100.18 (10.78) | 99.06 (11.02) | 0.72 |
| Salbutamol-induced changes in FVC (difference) [mean (SD)] | 1.41 (4.58) | 1.10 (4.94) | 0.82 |
| FEF25–75 at baseline (% pred.) [mean (SD)] | 117.15 (26.01) | 117.59 (25.26) | 0.95 |
| FEF25–75 post bronchodilation test (% pred.) [mean (SD)] | 121.35 (19.11) | 132.67 (25.76) | 0.10 |
| Salbutamol-induced changes in FEF25–75 (difference) [mean (SD)] | 7.00 (18.87) | 15.94 (14.35) | 0.04 |
In the group of T1DM patients, Hb A1 C levels (%) were 8.34 (7.4, 9.4). Children with T1DM were older than control group.
Comparing the two groups of children, we found no statistically significant differences in both “at baseline” and after bronchodilation testing FVC, FEV1, and FEF25–75 values. However, changes in mean “post-bronchodilator” values of FEF25–75 (ΔFEF25–75) were higher in children with rhinitis alone, as compared with children with T1DM and allergic rhinitis [15.9 (14.4) and 7.0 (18.9), respectively; p=0.04] (Fig. 1).
DiscussionThis preliminary observation might support the hypothesis that T1DM could exert a possible “protective” role in preventing asthma occurrence in children with also allergic rhinitis. In fact, the main finding showed that there was a significant difference concerning the FEF25–75 changes, such as the ΔFEF25–75, between the study group and the control group. In other words, only children with allergic rhinitis alone had a significant FEF25–75 increase after bronchodilation, whereas T1DM children had no spirometric change after β2-adrenergic administration. Two main concepts support the interpretation of these data. Firstly, starting from the assumption that reversibility to bronchodilation testing is considered the pathognomonic marker for asthma, it was previously demonstrated that a positive response to bronchodilation may be considered suggestive for subclinical asthma in patients with allergic rhinitis.14 Secondly, an impaired FEF25–75 value is actually considered a reliable marker of bronchial involvement in allergic rhinitis.15 Therefore, the absence of any spirometric change after bronchodilation should exclude the suspect of asthma as evidenced in the study group. On the other hand, even though an overt reversibility was not observed in children with allergic rhinitis alone, a significant increase of FEF25–75 values could lead one to suspect a possible evolution to bronchial impairment that should be further confirmed. In addition, it has to be noted that no child reported symptoms of asthma in both groups.
There are several mechanisms that might explain why T1DM patients may be less affected by asthma.16 Indeed, an autonomic nervous system dysfunction, with a reduction in sensory neuropeptide release in the airways related to an increased inhibitory neuronal M2 muscarinic receptors function might be involved.17,18 On the other hand, in animal models of diabetes it was shown that insulin treatment was able to: (a) normalise M2 receptor function (and therefore the vagal-mediated hyper-reactivity)19,20; (b) reverse the down-regulation of eosinophil accumulation in the airways following allergen challenge19; and (c) re-establish the bronchial contraction associated with mast cell degranulation and histamine release.20 In addition, another possible explanation of the possible “protective” role exerted by T1DM on asthma occurrence may be the result of the clinical correct management of these patients, followed by well-trained personnel with counselling programmes and educational courses, which included constant controls on compliance and adherence to treatment, on metabolic balance, while physical activity programmes and in general adequate life-style were closely addressed.
The main limitations of the present study are: the very limited number of patients, partially justified by the low epidemiologic impact of T1DM and above all about the association with allergic rhinitis, and the absence of a follow-up of these children. Thus, a further study to address these questions is on-going.
In conclusion, this preliminary study could sustain the hypothesis that T1DM associated with allergic rhinitis might exert a protective effect of preventing the possible evolution in asthma.
Ethical disclosuresProtection of human subjects and animals in researchThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Patients’ data protectionConfidentiality of Data. The authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Conflict of interestThe authors have no conflict of interest to declare.
Contributi del “Cinque per mille dell’IRPEF - Finanziamento della ricerca sanitaria”, Ministero della Salute (Italian Ministry of Health, Rome Italy).