Knowledge of the domestic mite fauna and allergen levels is important for a correct diagnosis and treatment of mite allergy. Our objectives were to describe the domestic mite fauna in the region of Murcia, Spain, to quantify mite allergens in dust samples obtained from mattresses of this area and to assess the influence of geographical, climatic and dwelling factors.
MethodsDust samples were collected in a transversal descriptive study from mattresses of 51 patients who went to the Allergology Service, and from mattress of 81 neighbours or family members of these patients. A questionnaire about home environment was filled in and obtained by all participants. Mite identification was done by light microscopy and allergen determinations (Der p 1 and Der f 1) by monoclonal antibodies.
ResultsSixteen mite species were identified in the 132 dust samples collected. The most frequent species were Dermatophagoides farinae (36% of the samples), Dermatophagoides pteronyssinus (32%) and Tyrophagus putrescentiae (5.3%). There were significant differences among climatic regions. The coastal sector had greater mite abundance, being D. pteronyssinus more frequent and abundant than D. farinae. In inland areas D. farinae was the predominant mite species. Allergen levels correlated with the concentration of Dermatophagoides, with higher levels detected in coastal regions. Average annual temperature was the main outdoor factor that correlated with higher mite concentrations. Indoor main predictor of higher levels of mites was the presence of obvious signs of humidity in the home.
ConclusionThis study demonstrates the existence of a mite fauna dominated by D. pteronyssinus and D. farinae with a strong influence of climatic factors and residential characteristics.
During recent years, there has been rising interest in the study of the domestic mite fauna in areas with high prevalence of allergic respiratory diseases or positive skin prick test to mites. This fact, associated to previous published studies demonstrating significant differences in the allergenic profile of sensitisation depending on the area of exposure,1 are promoting detailed studies in allergen content and mite fauna, influenced by different variables such as climate conditions, geographical distribution, allergen exposure, style of life, etc. An excellent review of these studies has been recently published.2
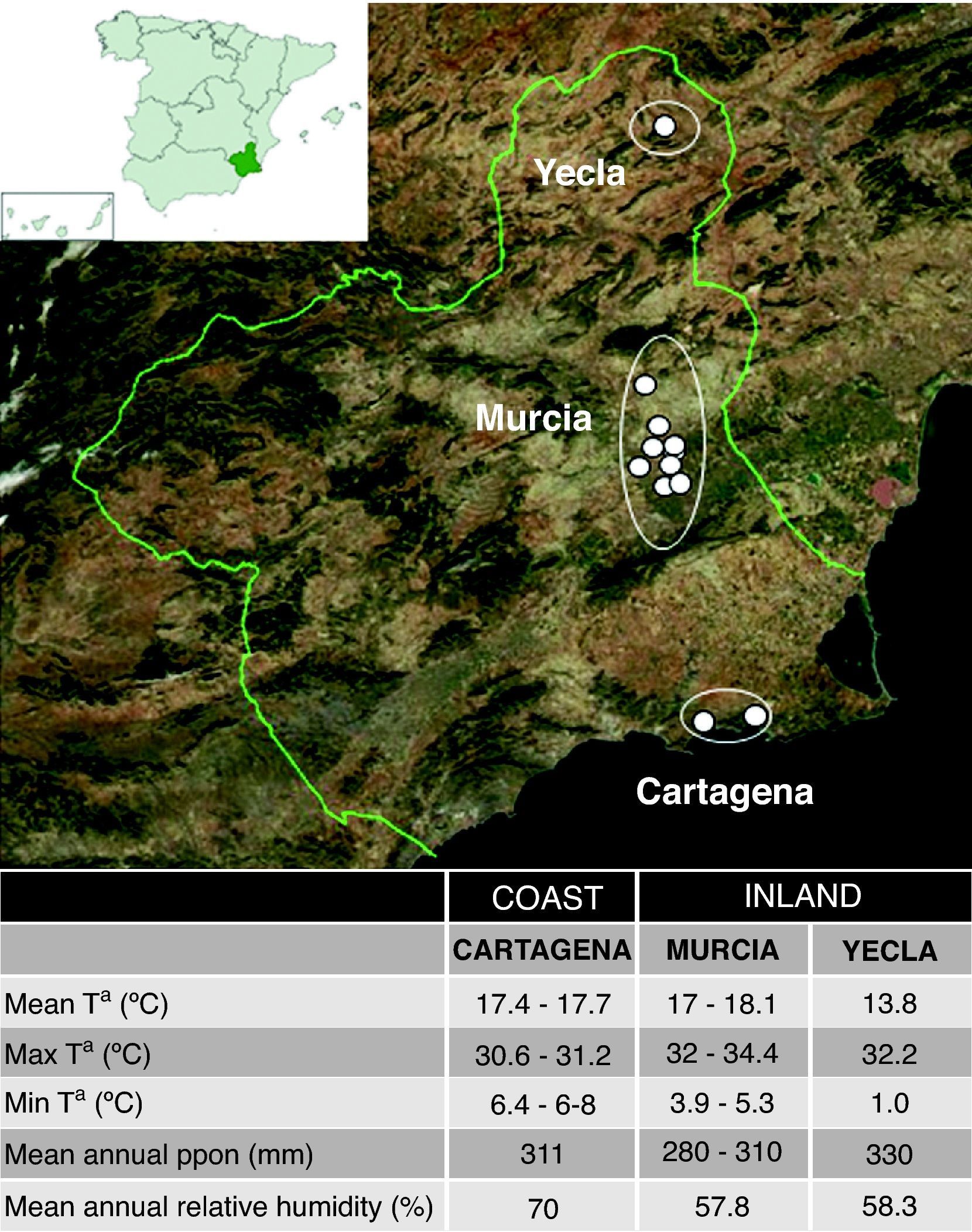
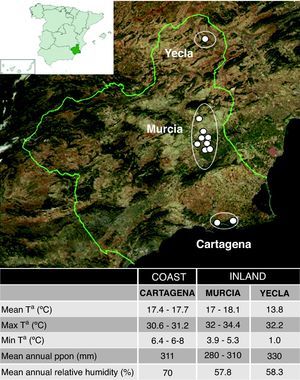
Murcia is a Spanish region located in the southeast of the Iberian Peninsula, between Andalusia and the Valencian Community, on the Mediterranean coast of Spain. It has a Mediterranean climate of semi-arid type, with mild winters and warm summers. The average annual temperature is around 18°C and the precipitations are scarce (about 300–350mm/year). The distance to the sea and the relief cause climatic differences between the coast and the inland (and among inland areas) mainly in winter. While the temperature on the coast rarely descends below 10°C, in the inland region it does not usually rise above 6°C and the precipitation level is significantly higher (up to 600mm/year).3
According to the data published in a recent survey about the prevalence of allergy and sensitisation in Spain, in the region of Murcia, 32.4% of patients suffering from rhinoconjunctivitis,4 and 38.1% of the asthmatic patients5 were sensitised to mites (mainly Dermatophagoides pteronyssinus and Dermatophagoides farinae). However, there is a lack of knowledge about the different mite species present in the region and their distribution. As for pollens, the identification of the main species is decisive for the correct diagnosis and treatment of mite allergy.6,7
The objectives of this study were to identify the domestic fauna in dust samples of mattress from different areas of the region of Murcia; to quantify Der p 1 and Der f 1 allergen levels; and to assess the influence of geographical, climatic and dwelling factors.
Material and methodsStudy populationDust samples were collected in this transversal descriptive study, during spring and autumn of 2004 and 2005, from mattresses of 132 participants living in region of Murcia. Fifty-one (38.6%) of these participants were patients who attended the Allergy Service, and 81 (62.4%) were relatives (who did not live in the same dwelling) or neighbours selected, independently of their allergic status, by the patients.
All participants filled in a questionnaire about their allergic status; sex; age; and home environment, including questions about habitat (rural, urban), dwelling type (house, floor), age of home, number of occupants, dampness (presence of obvious signs of humidity), pets, age of the mattress and pillow, heating and air conditioning. The allergic status was defined by affirmative responses to the question: ‘Are you suffering from allergic diseases?’ Information about allergy to mites was also recorded. However, since no specific allergological evaluation (symptoms, time of evolution, skin prick tests, specific IgE, etc.) of the participants was done to confirm their allergic status, that information was not included in the analysis. Climatic data of the localities were obtained from the Agricultural Geographic Information System (AGIS) of the Ministry of the Environment and Rural and Marine Environs. This agriculture GIS provides a meteorological model for each locality using meteorological data from 1960 to 1996. Relative humidity data were obtained from the State Meteorological Agency, using the annual average of the last 10 years.
In order to study the influence of the climate factors in the domestic mite fauna, we selected 99 participants living in three well climatologically characterised areas. Twenty participants lived in a coastal locality, Cartagena and surroundings, with a Mediterranean climate and a high relative humidity (70.4%). Another 52 lived inland, in Murcia city or surroundings, with a climate similar to the coast but with lower relative humidity (57.8%), and 27 were living in a relatively high altitude inland locality, Yecla, with a more continental climate and low relative humidity (58.3%). The location of these areas and their main climatic characteristics are shown in Fig. 1.
Dust samplesIndividuals who went to the Allergy Service were asked to collect a dust sample from their own mattress and two more from the mattresses of a neighbour and of a relative who did not live in the same dwelling. Dust samples were collected using three portable vacuum cleaners (1200W; Model Fagor VCE-300) modified to retain the dust sample in a coffee filter. The whole surface of the mattress was vacuum cleaned during 2min. Collected samples were immediately frozen to avoid mite proliferation. Once in the laboratory, dust samples, retained in the coffee filter, were weighed and separated in aliquots containing between 20 and 50mg, for mite identification and allergen determination.
Mite identificationMites were isolated using a suspension method. Briefly, 20–50mg of each dust sample was suspended in 10ml of a sodium chloride solution. Afterwards, an aliquot was poured into a Petri dish, examined and individual mites removed under a stereo binocular microscopy using a fine needle. The process was repeated with another Petri dish until all the volume was examined. The removed mites were placed one-by-one into a drop of Hoyer's medium and mounted on microscopic slides. Mites were counted, and identified, on morphological basis, using light microscopy by an expert acarologist. Results were expressed as number of mites per gram of dust.
Allergen quantificationAllergen levels, Der p 1 and Der f 1, were quantified using monoclonal antibody kits (Indoor Biotechnologies Ltd., Manchester, UK) according to the manufacturer's instructions. Results were expressed in microgram per gram of dust.
Statistical analysisDifferences between groups were calculated using the Mann–Whitney rank sum test and the ANOVA on ranks (Dunn's test) for multiple comparisons. The strength of association between quantitative variables was analysed using the Spearman rank order correlation test. Chi-square test (Yates correction) was calculated for comparison of qualitative data variables between groups. In all cases, P<0.05 was considered statistically significant. Statistical analyses were conducted using SigmaPlot, v.10.0 software (Systat, Port Richmond, CA, USA).
ResultsStudy populationTable 1 shows the personal and residential characteristics of all the individuals (132) who collected dust samples. No significant differences between participants who attended the Allergology Service and their family members or neighbours were observed, with the exception of the proportion of participants who reported to be allergic in each group (P<0.001).
Results of the questionnaire showing the personal and residential characteristics of the participants.
| Total individuals | Individuals who attended the allergology service | Family members/neighbours | P-value | |
| n | 132 | 57 | 75 | |
| Sex (%) | ||||
| Female | 53.5 | 56 | 51.9 | n.s. |
| Male | 46.5 | 44 | 48.1 | |
| Age (Mean±SD; years) | 38.4±19.7 | 33.1±16.5 | 41.7±20.9 | n.s. |
| Allergic (%) | 43.2 | 68.6 | 27.2 | <0.01 |
| Dwelling type (% homes) | ||||
| House | 35.8 | 34 | 36.8 | n.s. |
| Apartment | 64.2 | 66 | 63.2 | |
| Habitat (% homes) | ||||
| Rural | 30.1 | 29.2 | 30.7 | n.s. |
| Urban | 69.9 | 70.8 | 69.3 | |
| Age of homes (Mean±SD; years) | 24.9±14.2 | 23.7±14.6 | 25.7±14.1 | n.s. |
| Number of occupants (Mean±SD) | 3.8±1.3 | 4±1.3 | 3.8±1.3 | n.s. |
| Dampness (% homes) | 15.5 | 13.3 | 16.9 | n.s. |
| Pets (% homes) | 39.5 | 44.7 | 36.1 | n.s. |
| Age of mattress (Mean±SD; years) | 7.4±5.2 | 7.6±5.9 | 7.2±4.7 | n.s. |
| Age of pillow (Mean±SD; years) | 5.7±5.2 | 6.3±7.0 | 5.4±3.7 | n.s. |
| Heating (% bedrooms) | 65 | 65.2 | 64.9 | n.s. |
| Central heating (% bedrooms) | 27.5 | 28.3 | 27.0 | n.s. |
| Air conditioning (% bedrooms) | 32.9 | 28.3 | 31.1 | n.s. |
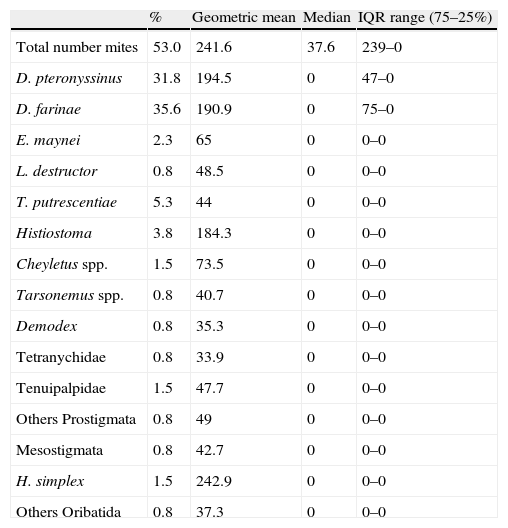
Prevalence and abundance of all mite species are shown in Table 2. Mites were detected in 53% of the dust samples collected, with a geometric mean population higher than 240 mites/g of dust. Fifteen different mite species were identified in dust samples. On 24.2% of the mattresses only one species was present, whereas in 28.8% two or more species were found. The maximum number of species found in one individual sample was four. D. pteronyssinus and D. farinae were clearly the dominant species with percentages higher than 30% of dust samples, and abundance superior to 190 mites/g (geometric mean) of dust. From all the samples containing Dermatophagoides, in more than half (50.9%) both species were present, in 20.3% only D. pteronyssinus was present, and in 28.8% D. farinae was the only Dermatophagoides species detected. In the samples where mites were detected, no statistically significant correlation between these two species (P=0.262) was obtained. The prevalence of Dermatophagoides mites is remarkable when compared to the other mite species, since the following species in abundance was present in only 5% of the dust samples (Tyrophagus putrescentiae).
Presence (% positive samples) and abundance (number of mites/g) of domestic mite species in mattresses from Murcia.
| % | Geometric mean | Median | IQR range (75–25%) | |
| Total number mites | 53.0 | 241.6 | 37.6 | 239–0 |
| D. pteronyssinus | 31.8 | 194.5 | 0 | 47–0 |
| D. farinae | 35.6 | 190.9 | 0 | 75–0 |
| E. maynei | 2.3 | 65 | 0 | 0–0 |
| L. destructor | 0.8 | 48.5 | 0 | 0–0 |
| T. putrescentiae | 5.3 | 44 | 0 | 0–0 |
| Histiostoma | 3.8 | 184.3 | 0 | 0–0 |
| Cheyletus spp. | 1.5 | 73.5 | 0 | 0–0 |
| Tarsonemus spp. | 0.8 | 40.7 | 0 | 0–0 |
| Demodex | 0.8 | 35.3 | 0 | 0–0 |
| Tetranychidae | 0.8 | 33.9 | 0 | 0–0 |
| Tenuipalpidae | 1.5 | 47.7 | 0 | 0–0 |
| Others Prostigmata | 0.8 | 49 | 0 | 0–0 |
| Mesostigmata | 0.8 | 42.7 | 0 | 0–0 |
| H. simplex | 1.5 | 242.9 | 0 | 0–0 |
| Others Oribatida | 0.8 | 37.3 | 0 | 0–0 |
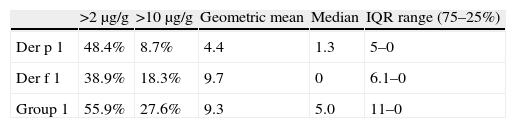
Mite allergen levels and presence are shown in Table 3. More than half of the dust samples had levels of group 1-mite allergens higher than 2μg/g, and more than a quarter exceeded 10μg/g. In samples with detectable allergens, no statistically significant correlation was found between Der p 1 and Der f 1 (P=0.463). However, significant correlations were obtained between Der p 1 levels and D. pteronyssinus populations, and between Der f 1 and D. farinae (P<0.0001)
Factors influencing mite and allergen abundancePersonal factorsNo statistically significant differences in mite and allergen abundance were found between the group of participants who attended the Allergology Service and their family members or neighbours.
No personal characteristic (allergic status, sex, age) showed statistically significant relationship with allergen or mite prevalence or levels.
Climatic factorsSignificant negative correlation (P<0.001) between altitude of the localities and Der p 1 and Der f 1 levels, D. pteronyssinus and D. farinae populations were observed.
There was a positive and significant correlation between the average annual temperature and Der p 1 (P=0.004), Der f 1 (P<0.001), D. pteronyssinus (P=0.016) and D. farinae (P<0.002) populations.
Regarding the average minimum temperature of the coldest month of the year, there was a significant and positive correlation with D. farinae, D. pteronyssinus (P<0.001) and Der f 1 (P=0.005) levels, but not with Der p 1. With respect to the average maximum temperature of the hottest month of the year, there was a positive and significant correlation only with Der f 1 (P=0.005). The total annual rainfall was correlated only with Der p 1 levels (P=0.007). No significant correlations were observed between mean annual relative humidity and mite allergen levels or mite populations.
Residential factorsMites and their allergens were more abundant in rural environment than in urban habitat, but this difference was only statistically significant in the case of Der p 1 (P=0.042) and D. farinae (P=0.013)
The presence of obvious signs of humidity in dwellings was correlated (P<0.05) with Der p 1 and Der f 1 levels, D. pteronyssinus and D. farinae populations. The presence of central heating was associated with lower D. pteronyssinus populations (P=0.037) and Der p 1 levels (P=0.038). Air conditioning was associated with increase in Der p 1 and Der f 1 levels (P<0.001), but not with mite populations.
None of the above factors were significantly related with the others (P>0.05), except for the presence of obvious signs of humidity, which was negatively significantly related (P=0.015) with central heating.
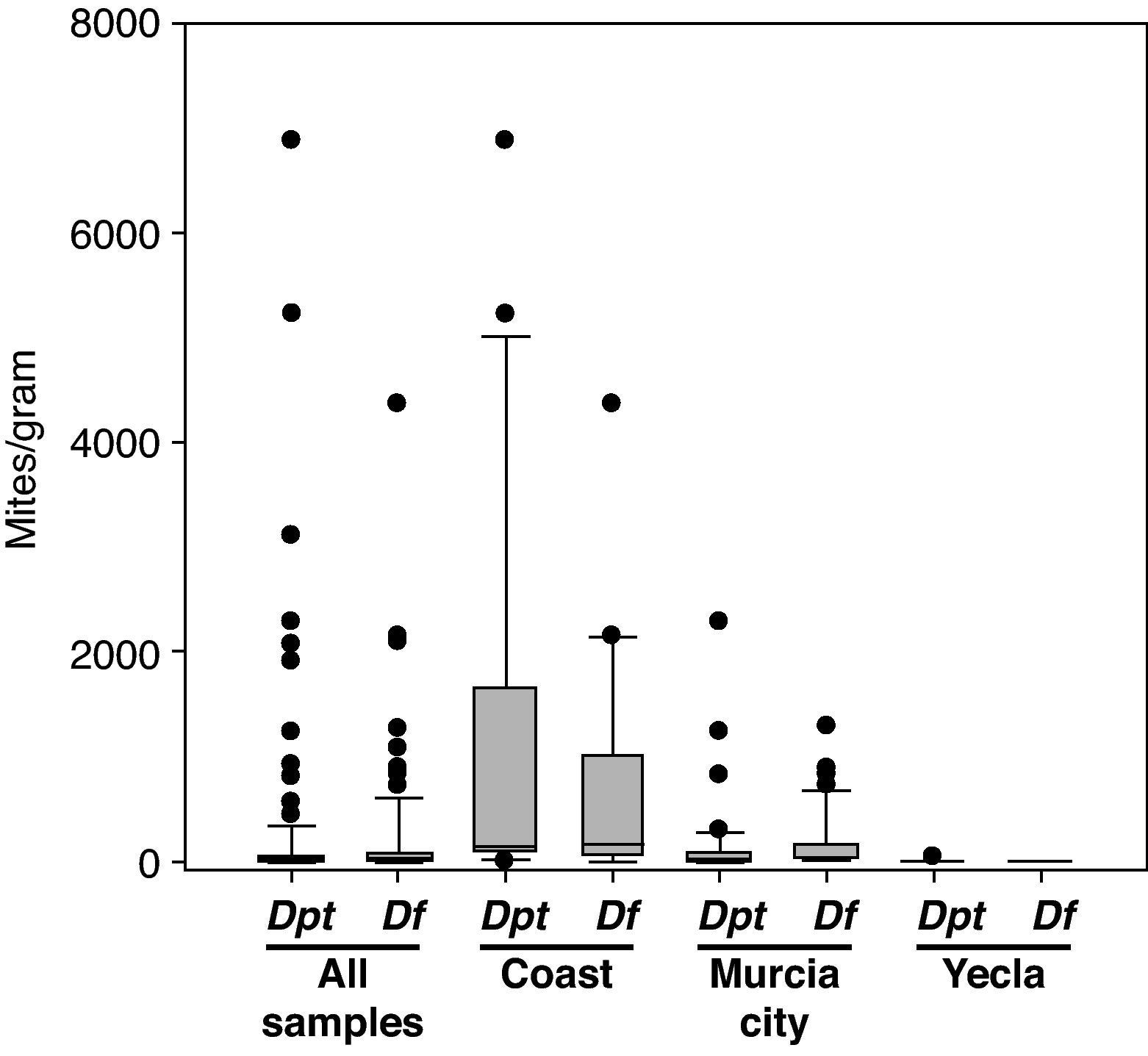
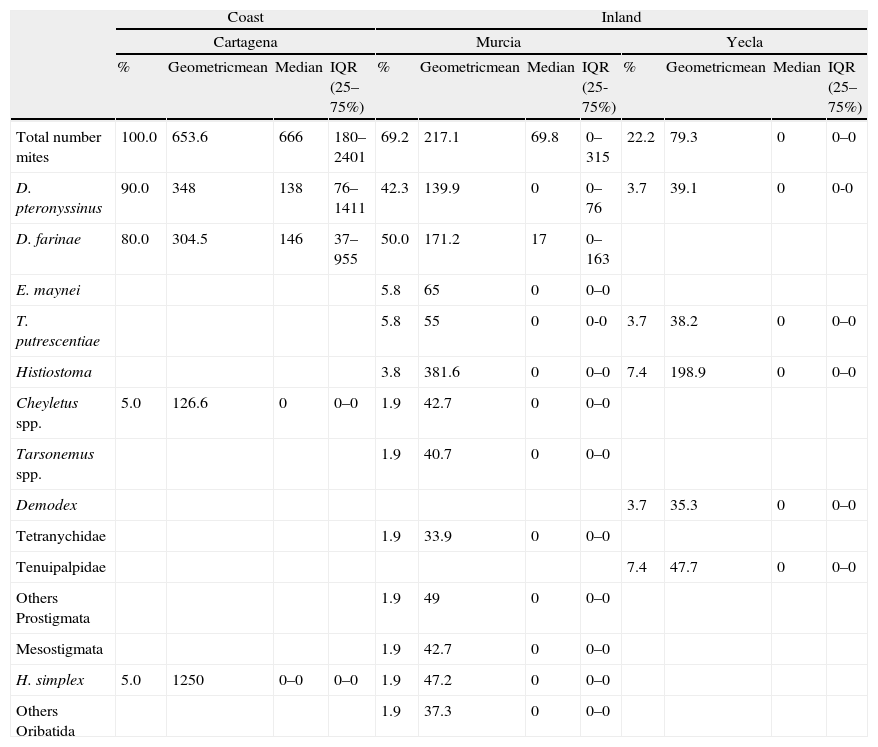
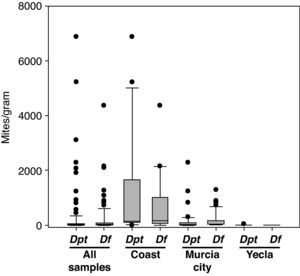
Climatic areasThere were important differences in mite presence and abundance among the three localities analysed (Cartagena, Murcia city and Yecla) (Table 4). The coastal area had a higher total number of mites, and D. pteronyssinus and D. farinae population (P<0.0001) than inland areas (except for D. farinae in Murcia city). Regarding interior areas, Murcia city and its surroundings had higher total mite populations and D. pteronyssinus and D. farinae levels (P<0.0001) than Yecla. There were no significant differences between D. pteronyssinus and D. farinae populations in any area.
Presence (% positive samples) and abundance (number of mites/g) of domestic mite species in localities of the region of Murcia.
| Coast | Inland | |||||||||||
| Cartagena | Murcia | Yecla | ||||||||||
| % | Geometricmean | Median | IQR (25–75%) | % | Geometricmean | Median | IQR (25-75%) | % | Geometricmean | Median | IQR (25–75%) | |
| Total number mites | 100.0 | 653.6 | 666 | 180–2401 | 69.2 | 217.1 | 69.8 | 0–315 | 22.2 | 79.3 | 0 | 0–0 |
| D. pteronyssinus | 90.0 | 348 | 138 | 76–1411 | 42.3 | 139.9 | 0 | 0–76 | 3.7 | 39.1 | 0 | 0-0 |
| D. farinae | 80.0 | 304.5 | 146 | 37–955 | 50.0 | 171.2 | 17 | 0–163 | ||||
| E. maynei | 5.8 | 65 | 0 | 0–0 | ||||||||
| T. putrescentiae | 5.8 | 55 | 0 | 0-0 | 3.7 | 38.2 | 0 | 0–0 | ||||
| Histiostoma | 3.8 | 381.6 | 0 | 0–0 | 7.4 | 198.9 | 0 | 0–0 | ||||
| Cheyletus spp. | 5.0 | 126.6 | 0 | 0–0 | 1.9 | 42.7 | 0 | 0–0 | ||||
| Tarsonemus spp. | 1.9 | 40.7 | 0 | 0–0 | ||||||||
| Demodex | 3.7 | 35.3 | 0 | 0–0 | ||||||||
| Tetranychidae | 1.9 | 33.9 | 0 | 0–0 | ||||||||
| Tenuipalpidae | 7.4 | 47.7 | 0 | 0–0 | ||||||||
| Others Prostigmata | 1.9 | 49 | 0 | 0–0 | ||||||||
| Mesostigmata | 1.9 | 42.7 | 0 | 0–0 | ||||||||
| H. simplex | 5.0 | 1250 | 0–0 | 0–0 | 1.9 | 47.2 | 0 | 0–0 | ||||
| Others Oribatida | 1.9 | 37.3 | 0 | 0–0 | ||||||||
Although D. farinae was more prevalent than D. pteronyssinus in the total number of samples, in the coastal area D. pteronyssinus was rather more frequent and abundant than D. farinae. Regarding inland areas, D. farinae was considerably more prevalent and abundant than D. pteronyssinus in Murcia, whereas in Yecla, the only Dermatophagoides mites found were D. pteronyssinus (Fig. 2).
Regarding storage mites, the most frequent and abundant non-pyroglyphid mite species in Murcia and Yecla area were Histiostoma spp and T. putrescentiae. In Cartagena, the only non-pyroglyphid mites identified were the predatory mite Cheyletus and the oribatid mite Haplochthonius simplex.
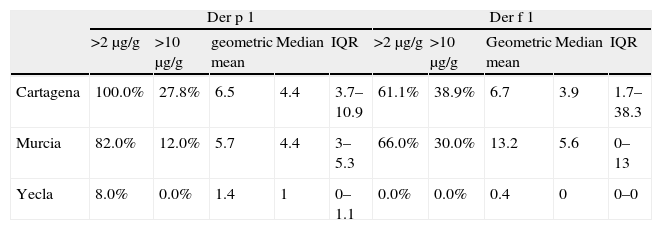
Regarding mite allergens (Table 5), the coastal area had higher Der p 1 and Der f 1 levels (P<0.001) than both inland areas. In these inland areas, Murcia city and its surroundings had higher allergen levels than Yecla.
Mite allergen prevalence (% positive samples) and levels (μg/g) in the dust samples in localities from the region of Murcia.
| Der p 1 | Der f 1 | |||||||||
| >2μg/g | >10μg/g | geometric mean | Median | IQR | >2μg/g | >10μg/g | Geometric mean | Median | IQR | |
| Cartagena | 100.0% | 27.8% | 6.5 | 4.4 | 3.7–10.9 | 61.1% | 38.9% | 6.7 | 3.9 | 1.7–38.3 |
| Murcia | 82.0% | 12.0% | 5.7 | 4.4 | 3–5.3 | 66.0% | 30.0% | 13.2 | 5.6 | 0–13 |
| Yecla | 8.0% | 0.0% | 1.4 | 1 | 0–1.1 | 0.0% | 0.0% | 0.4 | 0 | 0–0 |
In spite of the fact that in Cartagena and Murcia there are more percentage of mattresses with levels higher than 2μg/g of Der p 1 than of Der f 1, the percentage of mattresses with more than 10μg/g, and the mean levels, is clearly higher in the case of Der f 1 than of Der p 1.
DiscussionThe objective of this paper was to evaluate the degree of mite allergen exposure through the determination of mite allergen levels and the acarologic analysis of dust samples in a Mediterranean region (Murcia). The results show a domestic mite fauna dominated by D. pteronyssinus and D. farinae, with low presence of storage mites. According to our results, mite populations in Murcia are influenced by indoor dampness and outdoor climate. Both Dermatophagoides species have different ecological requirements and tend to predominate in different climatic areas. We have found significant differences in mite counts, species and allergen levels depending on the geographic areas and their climatic conditions. Patients living on the coast, and in the valley of the river Segura (Murcia city and surroundings), with a Mediterranean climate, are exposed to higher levels of Dermatophagoides and their allergens, than patients living in inland areas with more Continental climate influence (Yecla). Dampness in home is the main indoor factor associated with mite exposure in the region of Murcia.
Regarding the diversity of domestic mites in our samples, the number of identified species was clearly lower than those obtained in other Spanish regions,8 but similar to those observed in other Mediterranean regions like Catalonia.9 The evidence of humidity signs at homes was the only residential factor that correlated with the main mite species (D. pteronyssinus and D. farinae) populations, and their allergen levels. In spite of the fact that indoor relative humidity has not been recorded individually in our study, the presence of signs of dampness in the home can be considered as a sign of high indoor humidity. The negative relation observed between damp homes and central heating can confirm this hypothesis, since heating can reduce indoor relative humidity.10 The increase of indoor relative humidity has been previously associated with a higher prevalence of asthma, i.e. in the ecological analysis of the centres included in the ISAAC study,11 with higher mite allergen levels, i.e. Der p 1 in Europe,12 and directly with higher mite abundance13 (in spite of the fact that it does not affect all mite species in the same manner14).
Although domestic mites development depends on the indoor environment, this indoor climate is determined, in part, by the outdoor climate. Regarding outdoor temperature, we have observed a positive correlation between mites, and their allergens load with mean annual temperature, mean minimum temperature of the coldest month, and with the average maximum temperature of the hottest month (in this case only for Der f 1). Association between mean annual outdoor temperature and prevalence of asthma symptoms was observed in New Zealand adults,15 but it was not confirmed in a worldwide paediatric study,11 or in European adults.16 On the other hand, these studies showed a positive relation between asthma symptoms and lowest monthly mean temperature in European children11 and adults.16 However, association between outdoor temperature and mite population is not clear,2 because mite abundance is more dependent on the relative humidity (which depends, in part, on the temperature).
Nevertheless, we have not found a direct relation between annual relative humidity and mite and allergen load. The positive correlation observed between rainfall and Der p 1 can be not related with relative humidity, since there are many factors implied in the evaporation rate of the precipitation.2 On the other hand we have observed a negative correlation between mite populations and altitude, which is closely associated with humidity. This observation is in line with previous reports of inverse association between altitude and prevalence of asthma symptoms,11 and mite populations.17
Given the limitations of our study (absence of seasonal variation data, outdoor temperature and humidity obtained from long climatic series, and not from the time when the dust samples were collected), the associations of mite population with climatic factors would be related to the continentality. In this case, localities with more Continental climate influence (i.e. Yecla) with higher altitude, lower outdoor mean annual temperature and lowest mean minimum temperature of the coldest month (and probably with lower indoor humidity) had the lowest mite and allergen load. In comparison, low altitude localities with more Mediterranean climate influence (i.e. Cartagena, Murcia), with warm annual temperatures (higher outdoor mean annual temperature) and mild winters (higher mean minimum temperature of the coldest month) had high mite populations. Differences between Mediterranean and Continental climate with regards to mite presence and abundance, have been reported in other Mediterranean countries, i.e. Croatia18 or Israel,19 where, in spite of the fact that climatic disparities between localities made it difficult to compare the results, coastal localities globally had much higher dust mite populations and allergen concentrations than inland localities, as we have observed in our study.
The most remarkable finding of the mite fauna found in Murcia is the dominance of both Dermatophagoides species: D. pteronyssinus and D. farinae, with a great predominance over the other mites. Although we have not found statistically significant differences between D. pteronyssinus and D. farinae populations, there is a prevalence of D. pteronyssinus in the most humid area (coastal area), whereas D. farinae is the predominant species in the inland areas. This fact was confirmed with the allergen level determinations, since Der f1 levels increase significantly with the maximum temperatures, whereas Der p1 increase with the total annual rainfall. When these two species are found together in dust samples, frequently observed in European and American houses, one of them is usually dominant.20 Laboratory studies conducted in mixed-cultures showed that cultures dominated by one of the two species at the beginning of the study maintain the predominance throughout the experiment. However, D. farinae became dominant over D. pteronyssinus when cultures start with the same number of mites.21 Differences in microclimatic requirements explain the predominance of one species. In spite of that, these two species are very close taxonomically, there are differences regarding their response to environmental factors (i.e. higher optimum temperature for the development22 and resistance for desiccation21 in the case of D. farinae).
In general terms, and based on previous published studies, it is possible to confirm that D. pteronyssinus is the dominant species in Spain, especially in the North8 and in the Canary Islands, where humidity is very high. In the Mediterranean area there is an increase in the prevalence of D. farinae, as has been observed in other Mediterranean countries such as Israel23 or Italy.24 However, near the coast, where the humidity is higher because of the evaporation from the sea surface, D. pteronyssinus has a preponderance with respect to D. farinae, as has been observed previously in Spain (Valencian Community).25 Similar results have been observed in a comparable climate, as in California where D. pteronyssinus numbers were greater near the coast while D. farinae populations were higher inland.26
Regarding storage mites, the most common species in Murcia was T. putrescentiae, present in 5.3% of the dust samples, although this percentage is lower than those obtained in other Spanish regions, such as Huelva27 or Barcelona.28 The low presence of Lepidoglyphus destructor is striking, as is the absence of Glycyphagus domesticus because they are abundant storage mites in Spain.8,29 However, the ecological requirements of these species could be the reason, since these mite species prefer climates with less seasonal variation in rainfall and temperature.2 The non-appearance of Blomia is not surprising because this species has a tropical and subtropical distribution. Cross-reactivity or co-sensitisation to other mites could be the reason for the high prevalence of cutaneous sensitisation to this mite, observed in Cartagena.30
In conclusion, in this study we demonstrate the existence of a mite fauna in mattresses dust samples from the Autonomous Community of the region of Murcia, on the Mediterranean Coast, clearly dominated by D. pteronyssinus and D. farinae, and with low presence of storage mites. There are significant differences in mite counts, species and allergen levels depending on the geographic areas and their climatic conditions. Patients living on the coast and in the valley of the river Segura (Murcia city and surroundings) are exposed to higher levels of mites Dermatophagoides and their allergens than patients living in other inland areas with a more continental climate (Yecla). Dampness in home is the main indoor factor associated with mite exposure in the region of Murcia.
Conflict of interestThe authors have no conflict of interest to declare.