Melanoma differentiation associated (mda) genes in human encode a protein which has a surprising variety and diversity of interaction partners. It is a positive regulator of cancer cell progression in breast cancer, melanoma, and other human cancers. It regulates cell motility and invasion by altering defined biochemical and signalling pathways.
MethodsSuppressive subtractive hybridisation (SSH) has been done using a cDNA library prepared from lipopolysaccharides (LPS) stimulated and non-stimulated chicken spleen cells. Then PCR analysis and in situ hybridisation were done for further studies.
ResultsThis approach resulted in the identification of important chicken mda fragment. The obtained fragment was about 450bp covering the area from position 500 to position 950 of the human homologue. The expression analysis showed a wide variation in tissues and cell lines. In situ studies revealed mRNA expression in LPS stimulated tissues.
ConclusionIn this study a homologue for a chicken novel gene was described. The chicken melanoma differentiation associated gene-9 (mda-9) gene was found to be expressed in many tissues and cell lines in different levels. The stimulation time course was found to have a wide effect on both tissues and cell lines. The mda-9 gene was localised by in situ hybridisation and the effect of LPS stimulation was investigated.
As it is known that tumours had the ability to invade adjacent tissues and spread to distant organs, extensive research has been performed, expanding the body of knowledge concerning this basic hallmark of malignancy.1 Adapter proteins play an essential role in modulating signal transduction from the extracellular environment to the intracellular milieu by virtue of their association with key regulatory molecules.2 It was reported that the adaptor molecules and scaffold proteins are responsible for the organisation and assembly of multimeric protein complexes by driving the association of specific proteins with a variety of interaction domains, the integrity and specificity of a particular signalling pathway is assured.3 Syntenin is a PDZ-domain-containing protein that was originally identified as a potential mda gene, the expression of which was induced by interferon-γ (IFN-γ) treatment.4mda-9 was first cloned as a unique gene displaying biphasic expression during terminal differentiation of human melanoma cells treated with a combination of fibroblast IFN (IFN-γ) and the antileukaemic compound mezerein (MEZ).4,5 Terminal differentiation of human melanoma cells coincides with an irreversible loss of proliferative capacity, changes in biochemical programmes, alterations in surface antigen expression, modifications in cellular morphology, and major changes in gene expression. mda-9/Syntenin is an evolutionary conserved cytosolic protein representing a unique member of an expanding family of scaffolding proteins with highly potent and diverse biological activities. A notable feature of that protein is the presence of tandem PDZ domains of 83 and 80 amino acid residues, respectively (PDZ1 and PDZ2), which are required for the assembly and organisation of diverse cell signalling processes occurring at the plasma membrane.1,2 On a structural level, it is a 32-kDa protein that is made up of a 113-amino-acid NH2-terminal domain with no obvious structural motifs, followed by two adjacent tandem PDZ domains (PDZ1 and PDZ2) and a short 24-amino-acid COOH-terminal domain mda-9/syntenin has remarkable flexibility due to its ability for specific binding to internal or C-terminal sequences of target proteins.1,2 PDZ domains are reported to be ubiquitous signalling domains with more than 400 distinct copies in the human genome. This domain is composed of 80–90 amino acids, with a distinct fold of 6h-strands and two a-helices, and may occur in proteins harbouring other anchoring domains, but is also found in proteins that contain no other domains.3 This character allows mda-9/syntenin to participate in multiple biological functions including receptor clustering, protein trafficking, and activation of the transcription factor Sox4.1,2mda-9/syntenin was found to have the ability to induce morphological changes in cell shape in multiple cancers, including3,4 melanoma.4 Many research groups stated that mda-9/syntenin is co-localised with focal adhesion kinase (FAK) and facilitates FN-induced phosphorylation of FAK, with subsequent activation of p38 and c-jun NH2-terminal kinase mitogen-activated protein kinases.2 Syntenin was reported as an overexpressed gene in metastatic MDA-MB-435 cells. After that, investigations showed that there is a possible role of syntenin in metastatic progression of cancer cells. In this work, the chicken mda-9/syntenin homology fragment was obtained from chicken spleen SSH. The expression analysis and in situ localisation of this gene in chicken tissues and cell lines was examined. The data of this work may give more light on chicken mda-9/syntenin.
Materials and methodsTissues and cell linesBone marrow, brain liver, kidney, spleen, thymus, heart, lung and bursa of Fabricius were obtained from 12-week-old white leghorn HB15 antigen free chickens. Many cell lines such as macrophage cell line HD11,6 B lymphoblastoid cell line 1104B,7 chicken hepatoma cell line LMH,8 T lymphoblastoid cell line MSB19 and monocytic leukaemia cell line IN2410 had been biocultured in Iscove's modified Dulbecco's medium containing 8–10% foetal bovine serum (FBS). It was left to grow in bio-oven at 5% CO2 and 38°C.
Complementary DNA preparationTotal RNA is isolated from all tissues and cell lines used in this study, mRNA was purified from each by using (Fermentas). cDNA were reverse transcribed using Oligo-dT cDNA extraction kit (Promega). Chicken spleen SSH sample was prepared from chicken spleen cells stimulated by LPS 10g/ml (rough strain) from Salmonella typhimurium SL1181 (RE mutant) (Sigma, USA) in Iscove's medium for 3, 6, and 12h. The cDNA was prepared using PCR-select™ cDNA subtraction kit (Clontech, Heidelberg, Germany) according to the manufacturer's manual with a minor modification.
Preparation of expression analysis and PCR reactioncDNA for chicken mda-9/syntenin expression analysis was prepared from different tissues and cell lines by RT-PCR using the primers 5′-TCTCCAGCAT CTCTAGCTGG CC-3′ and 5′-TATACTCATAAATACTTGAAGG-3′ as forward and reverse primers, respectively, using chicken tissue and cell lines cDNA as a template. The PCR amplification was done using PTC-100™ according to the following thermal controller (MJ, USA). The PCR reaction was done using the following programme. The reaction mixture was incubated at 95°C for 10min, denaturised for 1min at 95°C, annealed for 30s at the optimal temperature which was decided to be 52°C and extended at 72°C for 2min. The reaction had been done for 32cycles and then finally incubated at 72°C for 10min for final extension.
In situ hybridisationFrozen sections of chicken tissues (spleen, thymus and bursa of Fabricius) for in situ hybridisation were performed using digoxigenin-labelled (Roche Applied Science) probes. Sense and anti-sense probes were prepared from partial chicken cDNAs obtained from SSH according to the manufacturer's manual with the following modifications: linearising the transcript (both sense and anti-sense) with T3 and T7/sp6 RNA polymerase. Frozen sections were fixed in 4% paraformaldehyde in 0.1% DEPC treated PBS for 30min and then in 0.1% active DEPC-PBS 15min 2 times for inactivation of RNase. After that, slides were immersed in DEPC-treated 5× SSC (0.75M NaCl, 0.075M Na-citrate) for 15min. Pre-hybridisation was performed at 58°C for 2h in 50% formamide/5× SSC buffer, 40μg/ml salmon sperm DNA. Hybridisation was then done for 4–40h at 58°C, with 400ng/ml probe of DIG-labelled chicken mda-9/syntenin fragment, in 50% formamide/5× SSC buffer, 40μg/ml salmon sperm DNA, in 50% formamide/5× SSC buffer saturated chamber. Tissues/slides were washed using 2× SSC buffer at RT for 1h at 65°C. Then washing for 1h in 0.1 SSC buffer and 5min equilibration in buffer-1 (Tris 100mM, NaCl 150mM, pH 7.5) were done. After that, slides were lifted O/N with anti-DIG antibody, Pod-coupled, diluted 1:200 in buffer 2 (buffer 1 with 0.5% Boehringer Blocking reagent) at 4°C. Hybridised slides were then washed 2× 15min in buffer-1. Slides were then equilibrated for 5min in buffer 3 (Tris 100mM, NaCl 150mM, Mgcl2 50mM, pH 9.5). Slides were stained with substrate kit for peroxidase (vector® NovaRED, Funakushi, Japan) for 30min and then washed with tap water for 15min and finally stained with methylene green for 5min, dried and mounted.
ResultsObtaining the subtraction fragmentThe subtraction clones were subjected to DNA sequence by using an automated applied bio-system model ABI-300 sequencing system. Data were analysed by homology search in the DDBJ/Gen Bank. The mda-9 cloned fragment was found to be about 450bp covering the area from position 500 to that of 950 down-stream of the human homologue. This clone showed 82.3% homology with the recently cloned human mda-9, accession number AF006636 (Fig. 1). That figure shows the aligned chicken fragment and human mda-9.4
Sequence comparison between chicken mda-9 fragment obtained from SSH and that of human and rat (from data base bank). (a) The text data alignment: the identical nucleotides among the three sequences were underlined by stars. (b) The picture data alignment: the identical nucleotides among the three sequences were enclosed in boxes.
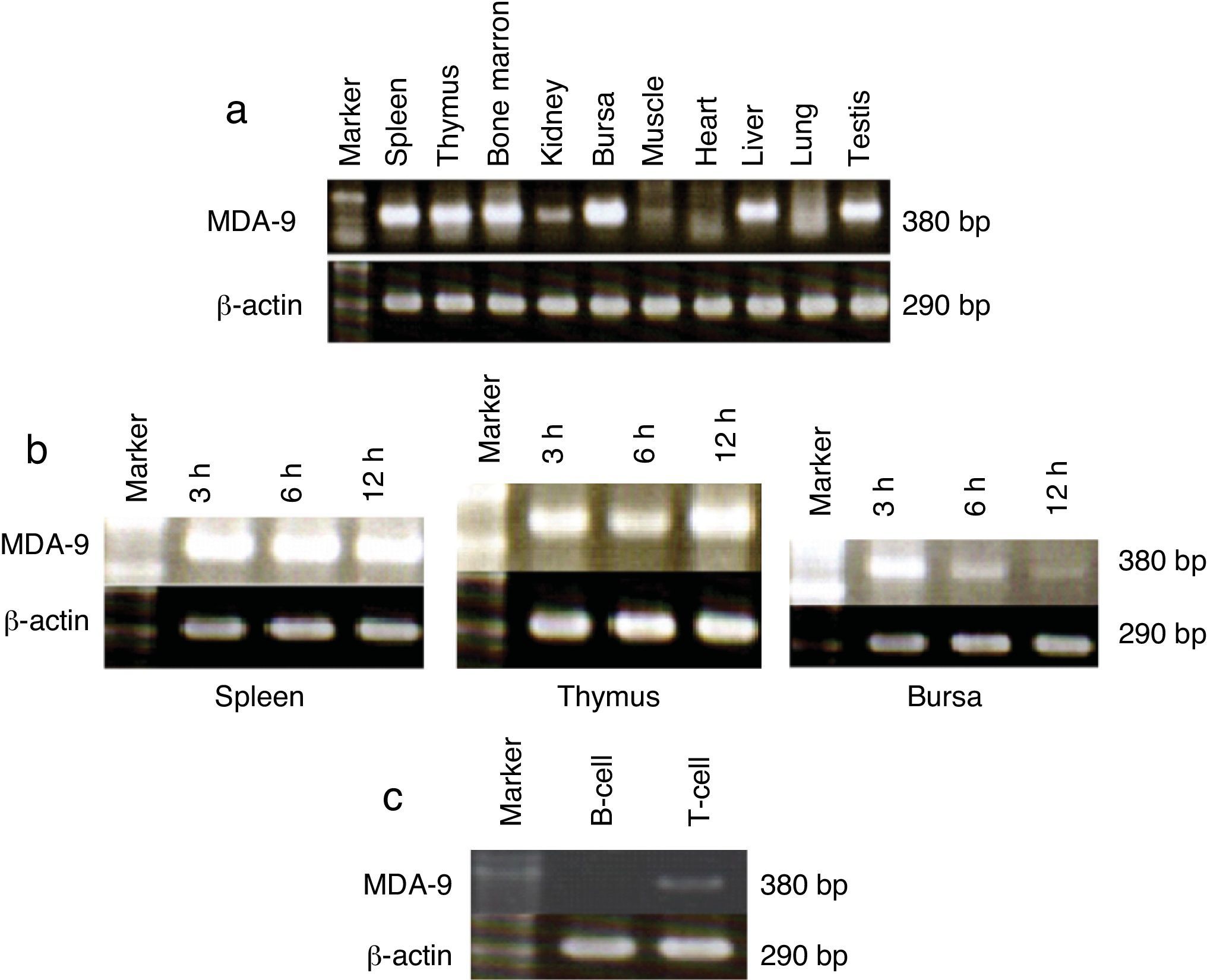
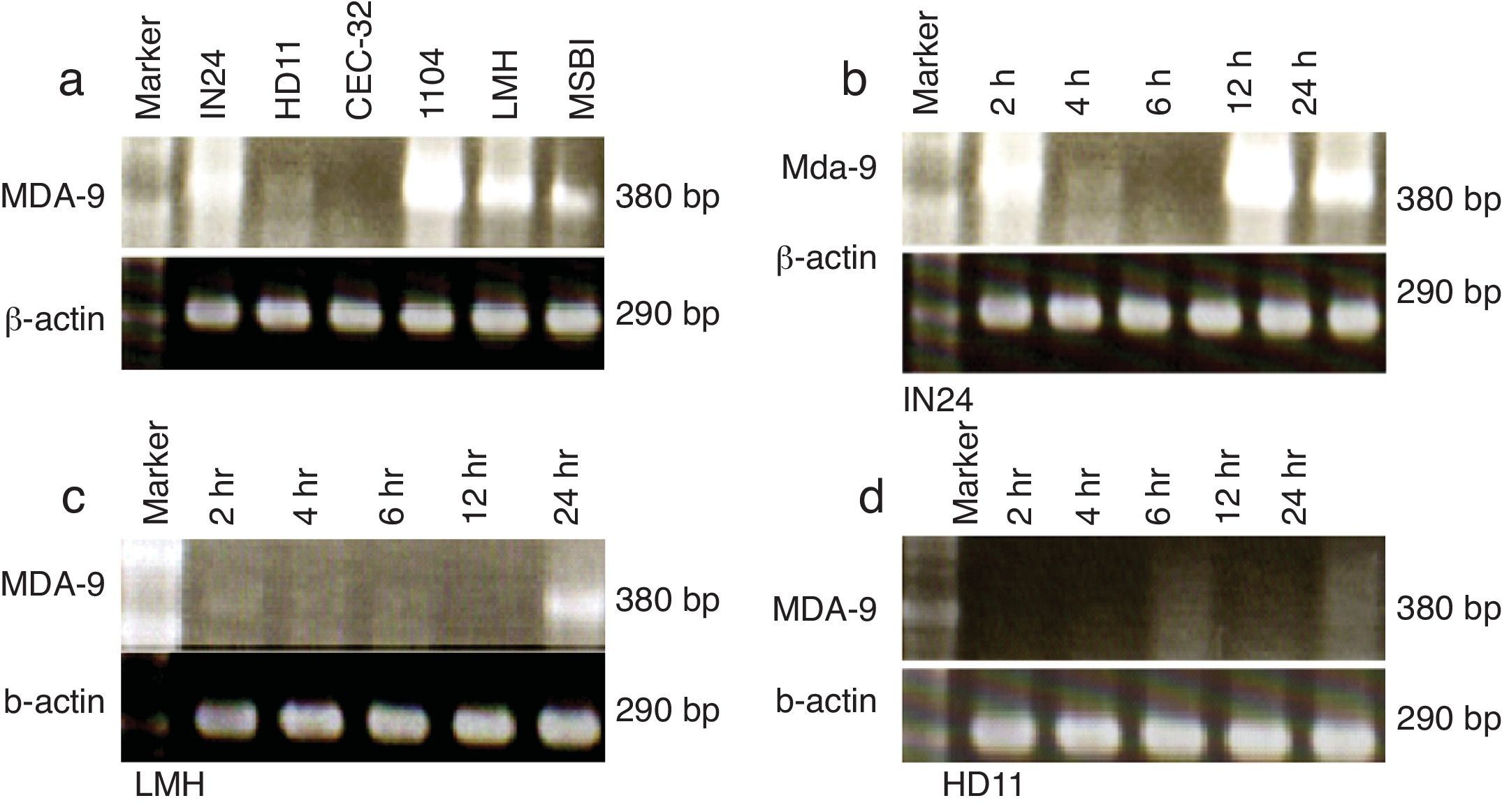
The profile of chicken mda-9 expression in different chicken tissues was shown in Fig. 2a, and the cell lines in Fig. 3a. The effect of time course of stimulation on this gene expression in organs (spleen, thymus and bursa) and cell lines (IN24, LMH and HD11) was shown in Figs. 2b and 3b–d. Chicken mda-9 showed a high expression in spleen, thymus, bursa, bone marrow, testis and liver and was low in kidney, lung, muscle and heart. The time course of stimulation showed no change in spleen; they decrease and then increase in thymus, but showed a gradual decrease of expression in bursa. The cell line examination showed a high expression in IN24, LMH, 1104B cell lines, low expression in HD11 and MSB1 and was not expressed in CEC-32 cell line. The expression analysis showed a decrease in IN24 cell line from 6 until 24h. In both LMH and HD11 the expression appeared to be increased by 24h stimulation (Fig. 3d) by fractionation of T and B cells. The chicken mda-9 was expressed in high level in T-cell in comparison with B-cell fraction.
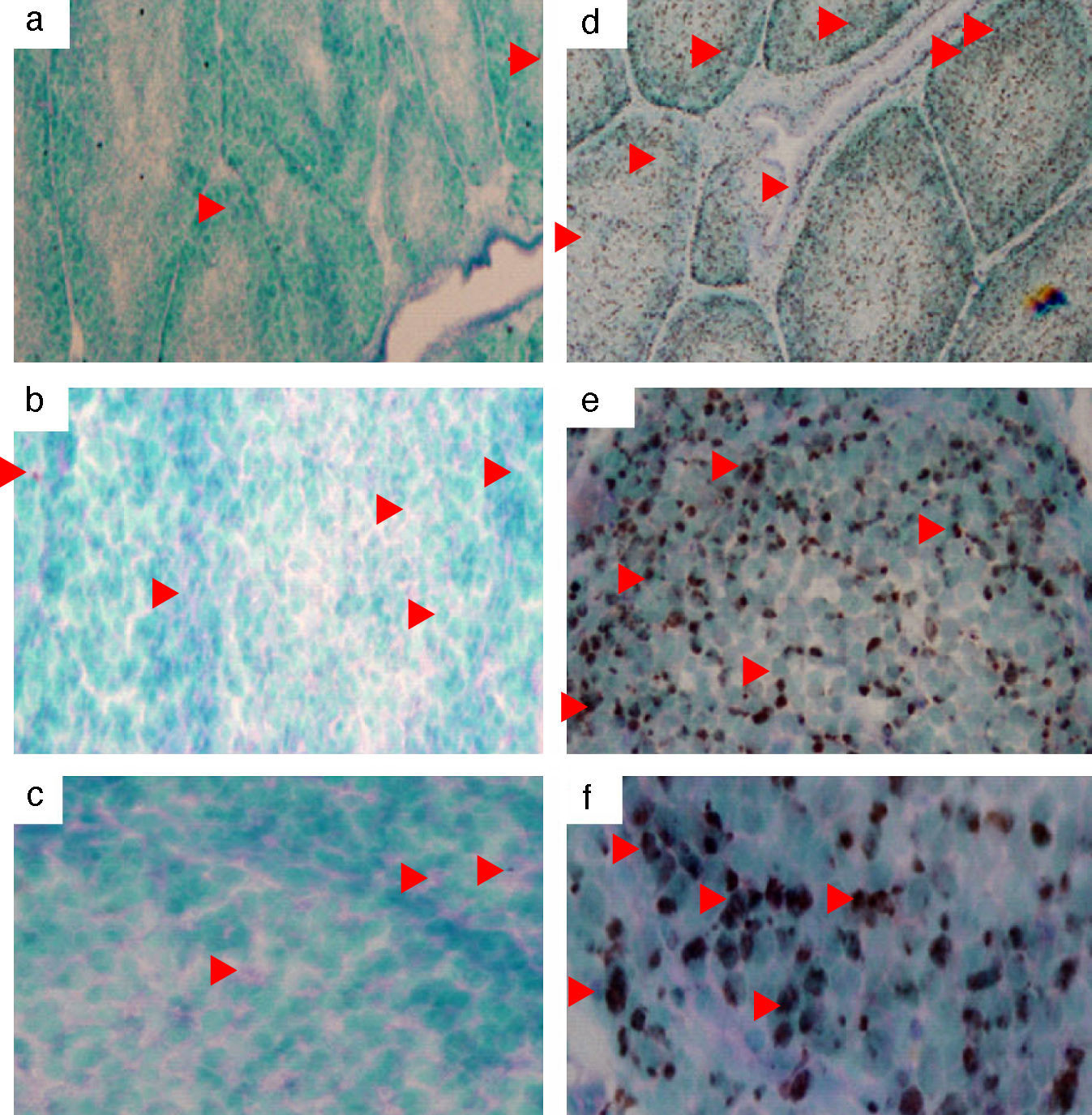
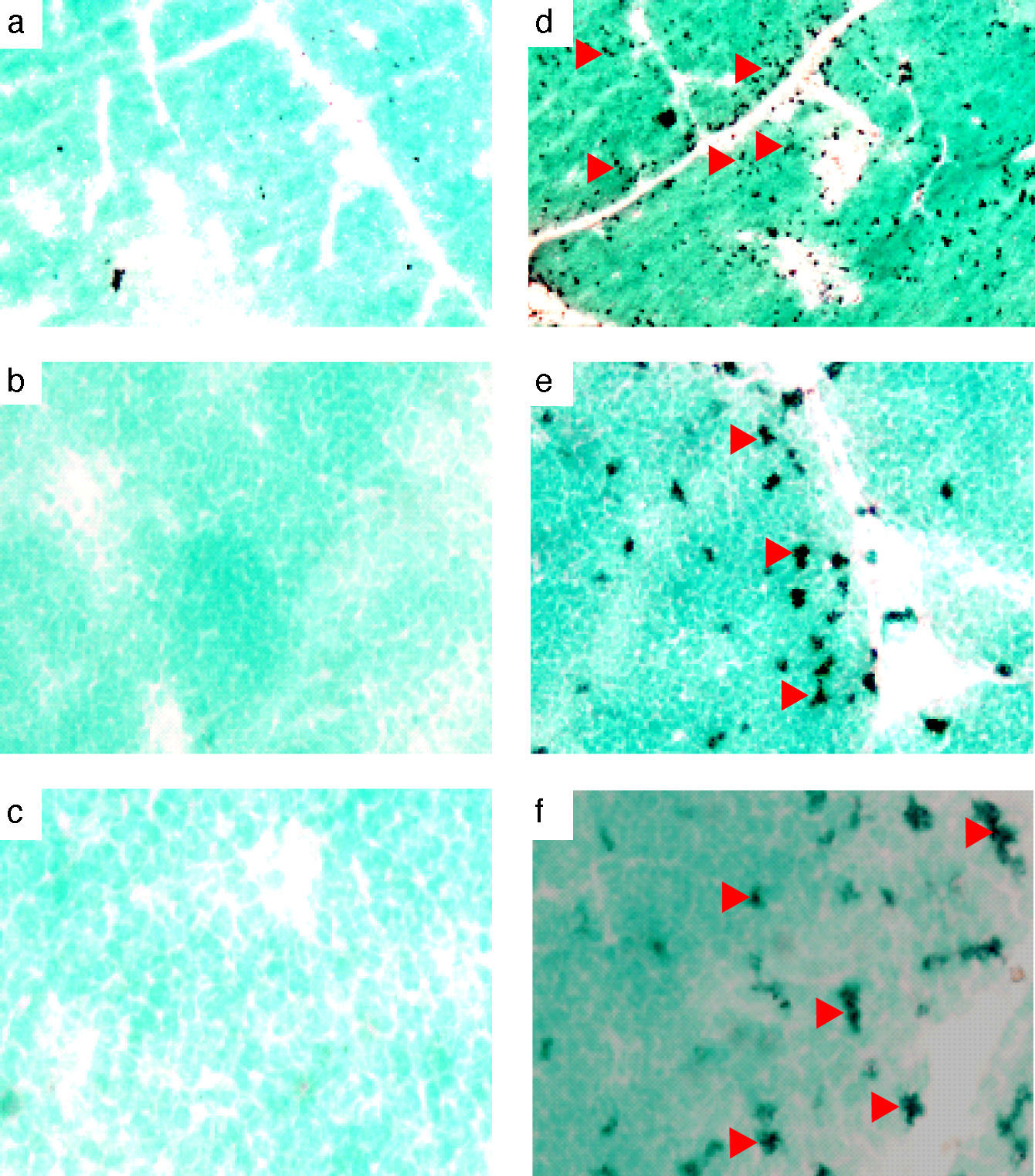
The chicken mda-9 was localised in chicken normal and LPS-stimulated spleen, thymus and bursa. Results of spleen stimulation showed that mRNA was increased due to LPS stimulation; the reaction was even found also in non-stimulated spleen (Fig. 6). In thymus, the mda-9 mRNA was increased in both cortex and medulla, Fig. 5. In bursa the reaction was high in all the lobules of bursa and the signal was very weak in the non-stimulated bursa (Fig. 4).
DiscussionCloning of chicken mda-9 fragmentSeveral different approaches utilising various methods have been undertaken to compare and identify gene expression related with the metastatic progression of human cancer cells. Among these approaches is subtraction hybridisation which represents an effective experimental method for identifying and cloning genes displaying differential expression. This strategy has been applied to chicken spleen cells stimulated with LPS. Many clones were analysed by the DNA sequencer and the clone that shows a homology with human mda-9 was selected to be subjected to further studies. We obtained a 450bp of chicken mda-9 homologue that showed more than 82% homology with that of cloned human mda-9 (accession number AF006636).4 The alignment of the SSH fragment with the nucleotide of human mda-9 found that the obtained fragment covered the position from 500 to that of 950 taking in mind the 5′ upstream un-translated region (Fig. 1). Forward and reverse primers had been designed to amplify the chicken SSH fragment by reverse transcriptase PCR for the expression analysis study.
Expression analysis of chicken mda-9Mda-9 has been reported to interact with a variety of receptors.11 The surprising diversity of mda-9-interaction partners suggests that it might have flexible cell-type-specific roles, forming unique scaffolds which are dependent on the intracellular environment or compartment in which it is localised. Thus far, most evidence points to a role for mda-9 in sub-cellular trafficking and signalling of receptors at the plasma membrane and within early endosomal and recycling compartments.11 Bearing these concepts in mind we can explain the diverse expression of chicken mda-9 in most different chicken organs as shown in Fig. 2a. Chicken mda-9 is expressed in high level in each of spleen, thymus, bursa, bone marrow and liver and also expressed in the rest of studied organs with respect to the difference in expression level in each one. The expression of mda-9 in all of these organs may be due to the flexible nature of that protein due to the presence of tandem PDZ domains of 83 and 80 amino acid residues, respectively (PDZ1 and PDZ2), which are required for the assembly and organisation of diverse cell signalling processes occurring at the plasma membrane.12,13 Although mda-9 was cloned first as a gene expressed in human melanoma cells, now it has been reported to be expressed in many cell lines and organs.12,13 In chicken it was found that mda-9 is expressed highly in many cell lines such as IN24, 1104B and LMH as shown in Fig. 3a. The chicken fibroblast cell line HD11 has been found to express low level of mda-9 mRNA while CEC-32 shows a very low expression level of mda-9. The LPS stimulation has been shown to have a remarkable effect on this gene expression in both organs and cell lines. In spleen, thymus and bursa of Fabricius, it showed different effects. In bursa the inhibiting effect was so marked as in Fig. 2b but was slightly so in spleen. In thymus the effect was different where the expression level was elevated, but to a small level (Fig. 2b). Among cell lines, three cell lines were checked for the effect of time course stimulation. In IN24 cell line the expression level was decreased until 6h after stimulation and again elevates its level. This result may be explained by the biphasic kinetics peaking from 8 to 12h after stimulation and returning to its normal level of expression after 24h of stimulation4,5 but this was not matched with the other two cell lines where, in both LMH and HD11, the expression level was increased gradually but in small grade (Fig. 3d).
In situ localisation of chicken mda-9A probe was prepared by labelling the purified RT-PCR product and then hybridised with each of frozen sections of chicken spleen, thymus and bursa of Fabricius. In all three organs the stimulation by LPS showed a remarked expression of chicken mda-9 mRNA (Figs. 4–6). It is reported that mda-9 has an important role in many cellular events5 where it belongs to the PDZ domain family of proteins and its specific localisation. One major recent role of mda-9/syntenin is its involvement in controlling tumour metastasis.12 The intense high signals in LPS stimulated organs may not be due only to chicken mda-9 where some studies reported that mda-9 is co-localised with phospho-FAK.14 However, a direct interaction between FAK and mda-9 was not reported, indicating that mda-9 might interact with other components of focal adhesion that regulate FAK phosphorylation.15–17 Although a multitude of interactions have been established, many of these interactions depend on overexpression systems, and clear functional effects have not been identified. mda-9 was also found to bind to the Wnt-receptor protein Frizzled 7.18,19
A recent report has revealed that although the expression of mda-9 in foetal tissue is abundant in the kidney, liver, lung, and brain, a much lower level has been detected in all adult tissues except the heart and placenta.20 Mda-9 expression level was elevated in invasive and metastatic human breast and gastric cancer cell lines relative to the level in poorly metastatic ones. Moreover, mda-9 expression level was elevated in gastric tumour tissues as compared with their normal counterpart.21 A detailed analysis of mda-9 expression in human breast and gastric tumour tissues could lead to an enhancement of our understanding of the potential role played by mda-9 in cancer progression and may provide a suitable marker for invasive tumours.
From the time of human mda-9 discovery as a mda protein many years ago, many new and exciting findings have indicated its involvement in a myriad of cellular functions. Therefore, many interaction partners and abundant expression patterns of mda-9 in chicken will fuel future research in this exciting direction after complete cloning of chicken mda-9. It will also be of great importance for our understanding of how chicken adaptor molecules organise intracellular protein complexes.
Ethical disclosureProtection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this investigation.Right to privacy and informed consent. The authors declare that no patient data appear in this article.Confidentiality of data. The authors declare that no patient data appear in this article.
Conflict of interestThe authors have no conflict of interest to declare.