Vernal keratoconjunctivitis (VKC) is a severe ocular disease with immediate and delayed hypersensitivity reactions which can produce loss of visual acuity and blindness. In its physiopathology a Th1 and Th2 response is present and the therapeutic approach is very difficult because the use of local immunosuppressive therapy such as topical corticosteroids for a long time could produce severe adverse effects. As with other allergy conditions, allergens can induce an immune response mediated through expression of IgE antibodies. Omalizumab is a monoclonal anti-IgE antibody indicated in severe asthma, but in recent years has demonstrated its usefulness in other allergic diseases as atopic dermatitis. We present a 16-year-old male patient with VKC with no response to conventional therapy but with important improvement in clinical symptoms after beginning omalizumab.
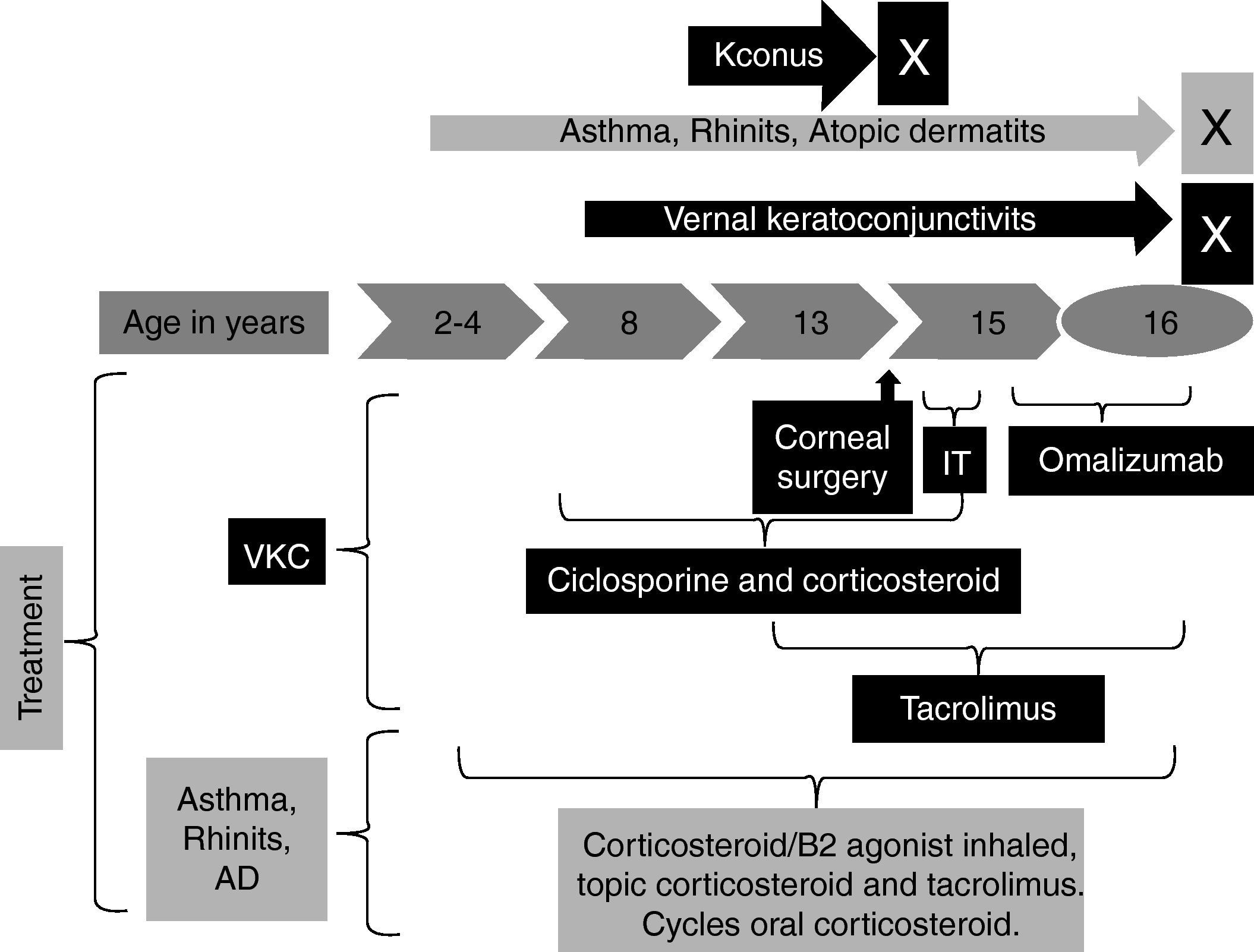
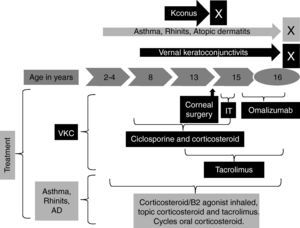
The patient began at two years of age with moderate atopic dermatitis and at four years of age with rhinitis and asthma. At the age of eight he presented severe ocular bilateral symptoms with red eyes, marked burning and itchy sensations, moderate photophobia, pseudogerontoxon, lacrimation, stringy discharge, papillae arranged in cobble stone and heaviness of eyelids. He received topical cyclosporine and corticosteroids in cycles for a long time without control. At the age of 13 he presented keratoconus in both eyes and required bilateral cornea transplant and permanent topical cyclosporine as immunosuppressive therapy for the transplant. At the moment it is his first visit to our allergy service. The patient had moderate atopic dermatitis with regular control with topical corticosteroids, lubricants and tacrolimus 0.1%; persistent moderate/severe rhinitis controlled with nasal corticosteroids and non-controlled asthma. We began tacrolimus 0.03% ointment for VKC and a combination of inhaled corticosteroids/B2 agonist for severe asthma with regular response for both therapies. The patient had total IgE 340UI/L and sensitisation to house dust mites (Der f, Der p) and we began immunotherapy but this was suspended after six months because of poor treatment adherence (Fig. 1).
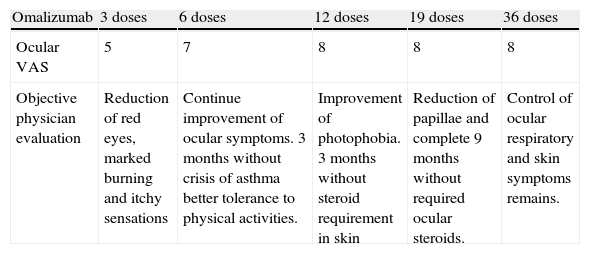
At age of 15 we decided to start omalizumab 300mg each two weeks for non-controlled asthma and VKC, ophthalmic and systemic immunosuppressive therapy was completely suspended and we only left tacrolimus 0.03%. After six weeks the patient presented an important improvement in ocular symptoms. After nine months he had a reduction of papillae. Other allergic conditions had an important improvement such as complete asthma control after six doses and atopic dermatitis after six months (Table 1).
Therapeutic responses to omalizumab.
| Omalizumab | 3 doses | 6 doses | 12 doses | 19 doses | 36 doses |
| Ocular VAS | 5 | 7 | 8 | 8 | 8 |
| Objective physician evaluation | Reduction of red eyes, marked burning and itchy sensations | Continue improvement of ocular symptoms. 3 months without crisis of asthma better tolerance to physical activities. | Improvement of photophobia. 3 months without steroid requirement in skin | Reduction of papillae and complete 9 months without required ocular steroids. | Control of ocular respiratory and skin symptoms remains. |
VAS: Visual Analog Scale. One is no control; ten is complete control.
Ocular allergies are categorised in seasonal or perennial conjunctivitis and vernal or atopic keratoconjunctivitis.1 Ocular symptoms normally run with asthma and rhinitis. Some articles describe ocular symptomatic changes in these patients following omalizumab treatment. Sotohi et al. observed a significant improvement in a group of seasonal rhinoconjunctivitis patients after a four-month period and one year after re-treatment.2,3 The same investigation group observed in a controlled study that the omalizumab patients group had less symptoms compared with suplast tosilate group.4
Additionally to conjunctivitis typical symptoms, atopic keratoconjunctivitis (AKC) and vernal keratoconjunctivitis (VKC) make conjunctiva and corneal damage which can lead to blindness. These diseases management is usually complicated and their pathophysiology is not totally clear but a Th2 and Th1 immunological response seems to be present.5 Clinical observation suggests that VKC generally subsides with the onset of puberty, but some therapeutic measures may be required beyond this age to control the course of the disease. In some cases, permanent changes to the ocular surface may occur and be accompanied by permanent visual impairment. New therapies such as tacrolimus have shown to be a promising alternative with good clinical efficacy and with less adverse effects than corticosteroids or other immunosupressors, however it is not always effective.6 Omalizumab could be a therapeutic option in patients with VKC refractory to conventional treatment; however to our knowledge few case reports have been performed studying the effect of omalizumab in VKC. Patricia Williams and John Sheppard described seven keratoconjunctivitis patients: three VKC and four AKC patients, who also had other allergic diseases such as asthma, atopic dermatitis and rhinitis. Six of the patients had a significant improvement of symptoms while one of the VKC patients did not show remarkable changes.7
Our patient had been receiving different immunosuppressive therapies for a long time for VKC and we only observed a significant response after he received omalizumab. Since this is only one patient and at the moment he received omalizumab he was also receiving tacrolimus we cannot be sure that the clinical response is not because of the combination therapy. However he had been receiving tacrolimus for almost two years with very poor response, and this suggests that improvement was more likely to be due to omalizumab than to tacrolimus. Our report together with Patricia Williams’ reports instigate to do more studies with an adequate methodological design to confirm omalizumab as an effective and safe therapy for VKC in patients with inadequate response to conventional therapy.
Finally there is the economical issue. Treatment with omalizumab is expensive and studies of cost–benefit analysis are scarce in asthma and there are none for other diseases. It can be argued cogently that success in exploring the effect of omalizumab as alternative therapy in other diseases different to asthma will lead to new methods for treating these diseases and preventing complications, with a decrease in overall healthcare costs in the long term.
Conflicts of interestWe don’t have any financial or personal relationship which could result in a conflict of interest with regard to the published article.