INTRODUCTION
At present, inhaled corticosteroids (ICS) are the first line medications for the treatment of asthma at all ages. However, even when ICS are very effective to control the disease, they need to be used as a long-term therapy where many aspects, mainly dependent on patients and treatments, are crucial to get the best possible clinical and functional benefits with minimal side effects, particularly in children. To achieve this goal simplified dosing regimens (e.g., once-daily administration) including good quality aerosols, good inhaler technique, close follow up of patients, and education of parents and patients on the importance of treatment adherence, are very important for the treatment effectiveness 1-5.
It has been reported that inhaling budesonide from dry powder inhalers (DPI) once daily is as effective as twice daily to control asthma in children and this may be an important advantage for parents and children in terms of cost, comfort and adherence to treatment 6-10. In addition, long-term treatment with budesonide once daily (200 or 400 µg) has been found to be safe and well tolerated in children and adults with newly detected mild persistent asthma 11,12.
Although there is a considerable body of information on once daily administration of budesonide DPI in children, little is known onto whether this effectiveness to control the disease can also be achieved by administering generic budesonide through MDI plus spacers in children. The latter would represent a cost-effective alternative, particularly in developing countries where DPI devices are still much more expensive than MDI and also may allow administering inhaled corticosteroids to patients unable to use DPI.
The present study was undertaken to determine the effect of once or twice daily administration of generic budesonide MDI plus spacer on asthma symptoms, spirometric lung function and airway responsiveness to methacholine, in children with mild-moderate stable asthma.
METHODS
Subjects
Fifty atopic asthmatic children (32 boys, 18 girls), with a mean age of 11 years (range 7-16 years), from a low income population and that were looked after at our Department of Pediatric Respiratory Medicine, Hospital El Pino, Santiago, Chile, were invited to participate in this study. They had mild to moderate persisting asthma, without exacerbations in the last 4 months, no history of acute respiratory infection in the last 4 weeks previous to randomization, and no systemic steroids in the last four months. All of them were on treatment with regular inhaled steroids up to 800 microgram daily of MDI beclomethasone dipropionate (or equivalent) and on-demand inhaled salbutamol; both delivered through a plastic opened spacer.
Study Design
This study was a randomized, single blind, two-groups, parallel, and 12-week clinical trial. During a four-week run in period children and mothers were trained on inhalation technique, forced vital capacity maneuvers and symptom recording (mainly wheezing and cough). At the end of the run in, children were randomly allocated into two study groups. One group inhaled budesonide MDI, 400 mcg bid and the other inhaled budesonide MDI, 800 mcg once a day in the morning. Salbutamol on demand was used for the relief of acute symptoms in both groups. MDI aerosols were inhaled by mouth using a large plastic spacer without valves. Instructions were given to mothers on cleaning the device with detergent to decrease electrostatic charge. The inhalation technique employed for all aerosols (budesonide and salbutamol) was as follows. After shaking canister, MDI aerosols were actuated into the spacer and slowly inhaled by mouth from residual volume to total lung capacity, holding breath for 10 seconds and then breath out slowly. Patients were instructed to rinse their mouth with water after inhaling budesonide.
Clinical assessment
Children were scheduled to visit our clinic every 30 days in a period of 12 weeks. At each visit, complete physical examination was done and parent-reported asthma symptoms (wheezing, on a yes or no bases) in the last 2 weeks before each visit were registered and employed for analysis.
Pulmonary function testing
Spirometric measurements were performed at entry (baseline) and at the end of the study using a heated pneumotachograph (model 3810, Hans Rudolph Inc. USA) with the Medgraphics CPF-S processing system (Medical Graphics Corp., MN., USA). Baseline values for FVC, FEV1, FEF25-75 % and FEV1/FVC were obtained prior to methacholine challenge, during each test day. Forced capacity maneuvers were done in triplicate and best spirometric values were selected according to ATS criteria for acceptability and reproducibility 13. Short-acting beta 2 adrenergic agonists were stopped 12 hours prior to lung function testing and inhaled steroids were allowed as prescribed by study physicians. None of the patients was on long acting adrenergic agonists, oral steroids, antihistaminics, or theophyline.
Methacholine challenge test was carried out if patient's FEV1 was equal or above 80 % of predicted value 14 and according to a modified tidal breathing method 15,16. Subjects performed all maneuvers in the standing position and using nasal clip, Methacholine chloride (ICN Biomedicals Inc., Ohio, USA.) solutions in normal saline were stored at 4° centigrade and nebulized at room temperature for 2 minutes using a Hudson 1730 updraft 2 nebulizer with a fill volume of 2 ml, driven by air at a pressure of 344 kPa (50 psi.) and flow of 6 l/min. Under the mentioned operation conditions nebulizers had an output of between 340-360 mg/min. Methacholine aerosol was delivered through a mouth tube with volume extension piece.
Following inhalation of normal saline, doubling concentrations of methacholine from 0.03 mg/ml to 8 mg/ml were inhaled by quiet mouth breathing during 2 min. FEV1 was obtained 30 and 90 seconds after nebulization. Doubling concentrations were given every 5 minutes until a fall greater than 20 % of the post-saline value of FEV1 was obtained (PC20). Sequentially, four puffs (400 µg) of salbutamol were then given by a large volume valved spacer.
Adherence to treatment
Adherence to treatment was estimated from parents and children reports and also from canister weight. All MDI canisters provided to children in both groups were weighted before and when collected at each corresponding visits to measure adherence to treatment (medication canister weight).
Morning plasma cortisol
Fasten blood samples for plasmatic morning cortisol (between 8:00 and 9:30 hr AM) were obtained for all patients at the beginning (randomization) and at the end of the study; plasma cortisol was determined employing radio-immunoassay considering as normal values those ranging from 5 to 25 µg/dl.
Data analysis
The presence or absence of asthma symptoms between every visit (yes or no) as reported by parents was computed as a categorized clinical score for comparison between entry and discharge. Concentrations of methacholine were logarithmically transformed prior to all calculations and PC20 was then calculated by linear interpolation of the final two points by means of a computer program. The number of double log-concentrations was then calculated (by subtracting the initial logPC20 to final logPC20 and dividing the result by log2) and employed to assess the change occurred in bronchial responsiveness to methacholine and expressing it as doubling dose change in PC20; one or more DD change in PC20 methacholine was considered as significant for the purpose of the study 17. An increase of 1 DD of the trigger after treatment with inhaled corticosteroids meant that double the amount of the trigger was needed to achieve the same fall in FEV1.
Analysis of variance (ANOVA), parametric and non-parametric test for paired and independent samples used where appropriate for statistical analysis. The limit of statistical significance was set at p < 0.05 (two tailed) and results are expressed as mean and 95 % confidence interval (95 % CI).
The study was undertaken with the permission of the Hospital's Ethics Committee and full informed, written and signed consent were obtained from all parents.
RESULTS
Of the 50 children initially enrolled, 44 completed the study. Six children were withdrawn; three in each group, and the main reasons were unwilling to continue with the study and failure to attend one or more of the scheduled visits (methacholine challenge test). There were not significant differences between groups in height and weight; demographic, lung function and other characteristics of patients are summarized in table I.
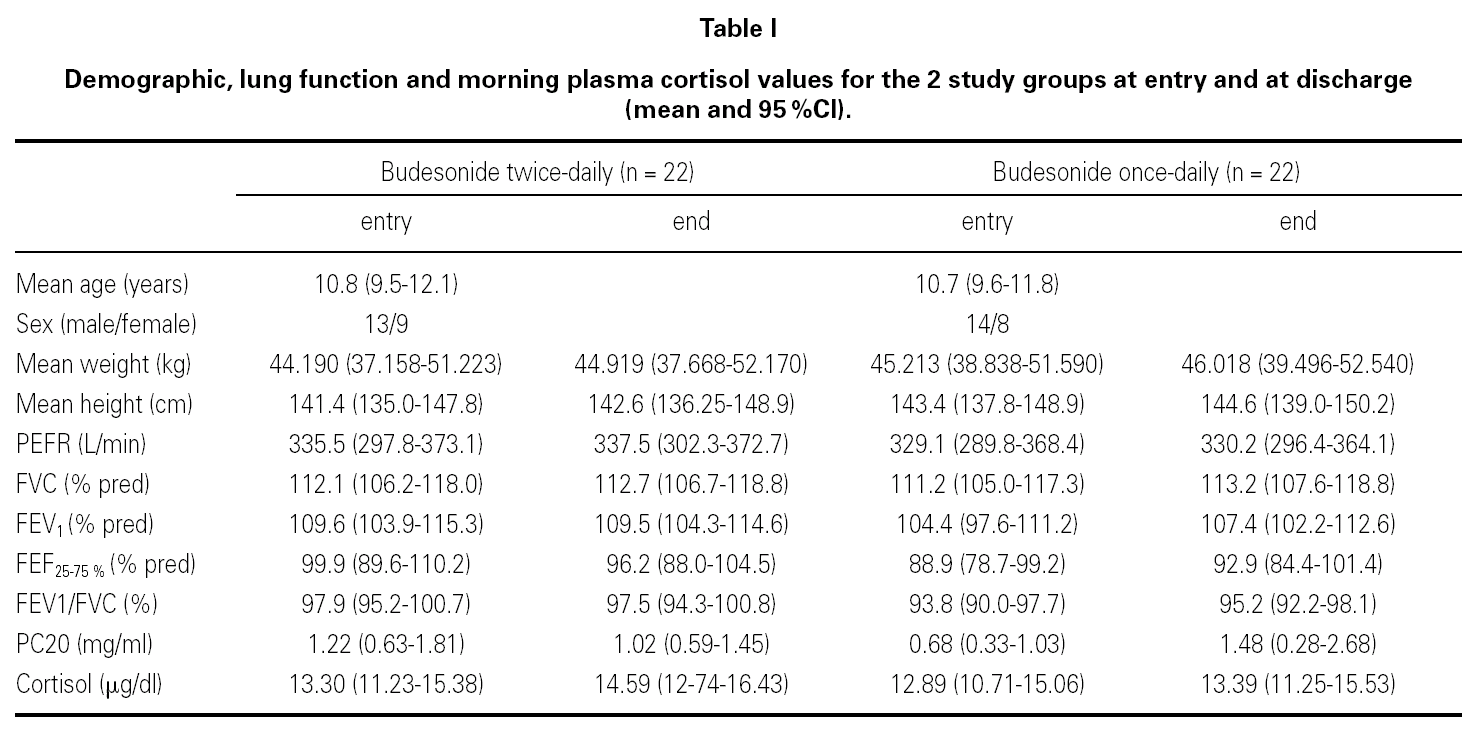
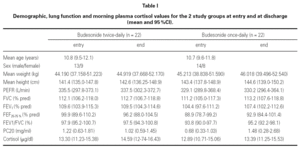
At the end of the study there was a significant clinical improvement in both groups, However, the proportion of children that still had asthma symptoms at week 12 was significantly lower in the group that inhaled budesonide once a day compared with the group inhaling twice a day, (chi-square 4.29, p = 0.038) (fig. 1).
Figure 1.--Reported asthma symptoms at entry and discharge in children treated during 12 weeks with budesonide 400 mg bid or 800 mg once daily (mean and IC95 %).
The general measured adherence (by canister weight) was significantly lower for the group of children that inhaled budesonide twice daily (62.9 %; 95 %CI 54.5-71.3) as compared with those who inhaled once daily (74.4 %; 95 %CI 68.9-79.9 %). In both groups parents and children reported adherence to treatment over 85 %, however, the agreement between reported and calculated was poor (kappa = 0.13)
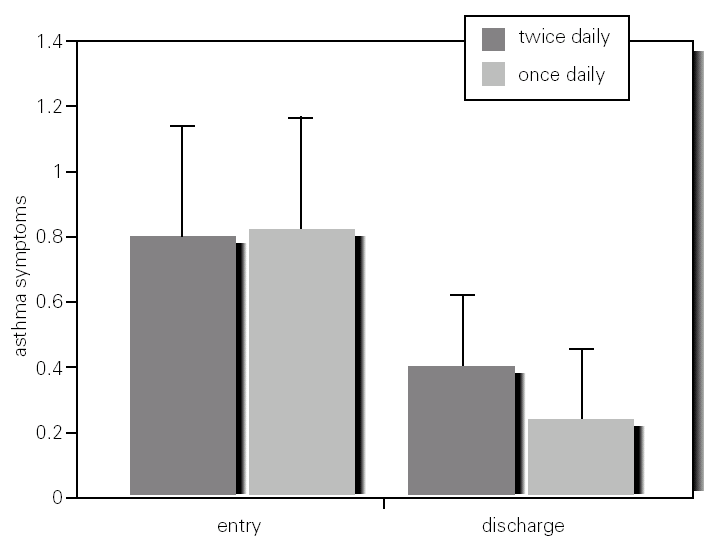
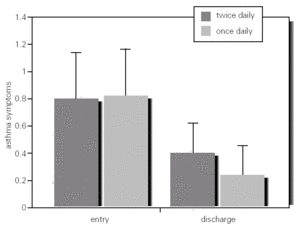
Spirometric and PEFR values between and within groups at baseline and discharge were not significantly different (table I; fig. 2).
Figure 2.--Mean PEFR and 95 %IC in asthmatic children who inhaled MDI budesonide 400 mg twice daily and 800 mg once daily, from entry to discharge (week 12).
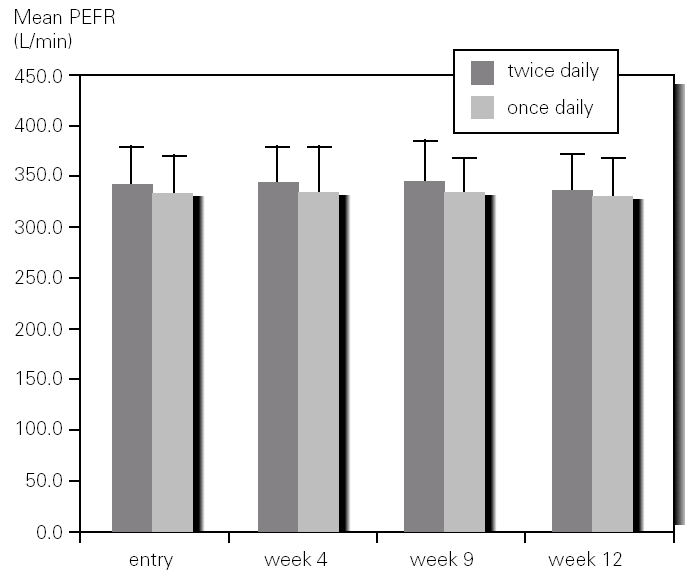
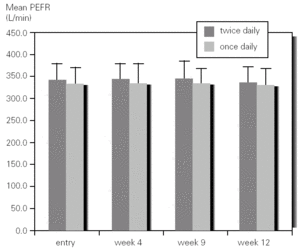
There was not significant difference in mean methacholine PC20 between entry and discharge for any of the study groups. Although the group of children who inhaled budesonide once daily showed an increase in mean PC20 from 0.68mg/mL to 1.48 mg/mL it did not reached statistical significance. However, when expressed as log PC20 doubling dose change there was a significant improvement (1 or more DD) in the once-daily group (1,22; 95 %CI, 0,60-1.85) as compared with the group that inhaled budesonide twice daily (0.46; 95 %CI, 0,23-1.14) (fig. 3).
Figure 3.--Doubling dose change of methacholine PC20 after 3 months of treatment with budesonide 400 mg twice daily (bid) or 800 mg once daily in asthmatic children.
Morning plasma cortisol was not significantly different between and within groups at entry or end of the study. There were no complications related with employed medications reported by patients or their mothers during the study.
DISCUSSION
This study shows that both, MDI budesonide given once (800 µg), or twice daily (400 µg BID) used for 16 weeks, are similarly effective to control asthma symptoms in children with mild to moderate asthma. However, once daily budesonide had a significant effect on improving BHR and was more effective in controlling asthma symptoms than administering the medication twice daily. The latter may be associated to the significantly higher adherence to treatment observed in the group of children who inhaled budesonide once daily.
These findings have been reported in the past using inhaled corticosteroids mainly administered by DPI in children and adults. In a randomized, double-blind, placebo-controlled, multicenter study which included 274 asthmatic children aged 6 to 17 years showed that budesonide turbohaler (200 µg or 400 µg) once daily for 12 weeks were similarly effective to control symptoms and to improve lung function as compared to placebo 18. Recently, a meta-analysis on the efficacy of budesonide administered once daily compared to twice daily in patients with mild to moderate asthma found that once-daily budesonide regimen has a similar efficacy to a twice-daily regimen in doses up to 800µg per day and the authors have suggested that once-daily regimen has potential advantages in terms of patient compliance and satisfaction 12. In a large prospective randomized study, the long-term (up to three years) once-daily treatment with DPI budesonide (200 µg to 400 µg) decreased the risk of severe exacerbations and improves asthma control in patients with mild persistent asthma of recent onset 11.
Despite improved treatment protocols, asthma continues to be associated with high rates of morbidity-mortality, and poor adherence to individual treatment plans is one of the more important factors for these poor outcomes. There is an increasing consensus on the crucial role of a good adherence to asthma treatment to get the expected clinical and functional control of the disease either in the daily medical practice as in research. However, accurate assessment of medication adherence is difficult to achieve because the several factors involved (patient and parents education, family context, prescriptions, comfort, etc) and also because there is not a definitive method to measure adherence. Despite difficulties to assess adherence, an effort should be made by clinicians and researchers to objectively assess it when prescribing asthma treatment to patients. It has been shown that there is a significant discrepancy between the adherences to MDI inhaled treatment reported by mothers or children (80 %) and the calculated adherence either by canister weight (69 %) or electronic dosimeter (50 %) 5. However, a low adherence also occurs when using inhaled steroids from a DPI with a dose monitor on the device (68 %) 19.
It has been demonstrated that risks for poor adherence predict subsequent asthma morbidity and that most of these risks can be controlled by physicians through reducing the complexity of asthma regimens, communicating effectively with caregivers about medication use, and correcting family misconceptions about asthma medication side effects 4.
The effect of inhaled budesonide on lung function in asthmatic children is rather controversial and two large long term controlled studies 11,20 have reported conflicting results. START 11 using budesonide turbohaler 200mcg once daily found a highly significant improvement in both prebronchodilator and postbronchodilator FEV1 % values after 1 and 3 years of the study for the treatment group as compared to placebo. However, in the CAMP 20 study continuous daily treatment with budesonide turbohaler 200mcg twice a day showed no significant effect on lung function, as measured by the FEV1 after bronchodilator use, as compared with placebo.
In the present study none of the employed modalities of inhaled budesonide had effect on changing lung function and that could be in part explained by the short term study observational time (3 months) and also because in both groups lung function was over 85 % predicted at the time they were randomized. In this regard there is some controversial information. Some authors have found significant improvement of lung function and symptoms in 3 months with DPI budesonide once daily as compared to placebo 18. Others using same delivery system and doses for long term have not found significant changes in lung function 20 in asthmatic children. A similar long-term study 11 reported that a highly significant improvement in both prebronchodilator and postbronchodilator FEV1 % values was observed after 1 and 3 years of the study for budesonide DPI treatment group compared with placebo.
In the daily practice is common to observe that many of the children with mild-moderate stable asthma have normal lung function and few symptoms, even when they could have BHR and eventually airway inflammation. It has been found that most asymptomatic asthmatics continue to exhibit BHR and signs of airway inflammation and the outcome of childhood asthma and BHR would be associated with the degree of airway inflammation and the duration of childhood asthma 21. A meta-analysis assessing the dose of inhaled corticosteroid and the minimum duration of treatment required to obtain a significant improvement in BHR found that high doses of inhaled corticosteroids (mean dose 1,000 µg, range 4002,000 µg daily) decreased BHR within 28 weeks in patients with corticosteroid naive asthma but remained unclear whether lower doses of inhaled corticosteroids can achieve the same results 22.
It is well known that every asthmatic patient should receive his/her own treatment modality destined to control the symptoms, to improve pulmonary function and decrease bronchial hyperresponsiveness and the use of rescue medication to a minimum. Thus, an important issue to consider for asthma treatment is that the dose of inhaled steroids needed to improve symptoms, improve peak flows, reduce beta-2 use, improve FEV1, improve BHR, and prevent severe exacerbations is likely to be different. It has been demonstrated that even when low and high dose of inhaled steroids had similar effects on symptoms and peak flows, the higher dose was markedly more effective at reducing severe exacerbations 23. The dose employed in this study (800 µg per day) is well in the range of those reported to be effective to control symptoms and improve BHR 12,22 and it was safe and well tolerated in both groups.
The satisfactory clinical effect on asthma symptoms with once daily administration of budesonide through MDI plus spacer, as found in this study, may represent an advantage in terms of treatment compliance for asthmatic children, cost-effective alternative and to facilitate the long term treatment with inhaled corticosteroids in younger children who are unable to inhale medications from DPI.
Conclusion, in this study, once daily administration of 800 µg of inhaled budesonide administered by MDI plus spacer was better in controlling symptoms and improving BHR than fractioning the dose to 400µg twice daily. The higher adherence to treatment of patients from once-daily inhaled budesonide could have accounted for the observed differences.
Correspondence:
Prof. Dr. Javier Mallol
PO Box 23-Correo 9
Santiago, Chile
Fax: 56 2 331 0375
E-mail: jmallol@usach.cl
Grant n.º 83-20.00691. Department of Scientific and Technological Research (DICYT). University of Santiago de Chile (USACH).