We aimed to determine the frequency of oropharyngeal candidiasis and its clinical correlates in the asthmatic patients who use fluticasone propionate (FP) as a dry powdered inhaler.
We selected four groups of patients: 62 asthmatic patients who were taking 200 μg/d FP, 122 asthmatics who were taking 500 μg/d FP, 50 asthmatic patients who had not been on inhaled corticosteroid (ICS) treatment and 40 normal non-asthmatic subjects.
The frequency of positive swabs for Candida colonization was higher in 500 g/d FP group than asthmatics without ICS use (χ2=6.8, p<0.05) and normal controls (χ2=4.9, p<0.05), whereas it wasn’t different in the 200 g/day FP group when compared to controls. When we considered patients who used ICS, the most effective variables affecting the occurrence of Candida colonization were washing of the throat by the patients (OR=9.4, 95 % Confidence Interval [CI]=3.9-22.7, p<0.0001) and duration of ICS use more than 12 months (OR=2.5, 95 % CI=1.1-2.6, p<0.05).
The present study showed that in the patients who use ICS, the most important determinants on colonization were not washing the throat regularly and duration of ICS use for more than 12 months.
Inhaled corticosteroids (ICS) have the properties of low systemic potency coupled with a high topical antiinflammatory activity. Among the local side effects, hoarseness and oropharyngeal candidiasis are the most common and clinically limiting factors. These side effects occur due to the deposition of the drug in the oropharynx. The incidence of oral candidiasis has varied from 1 % to 77 % with ICS treatment probably because of the method which is used to detect it1,2. It is well known that the rates of colonization and infection are different entities. For example, Candida infection was reported as 22 %, whereas colonization was reported as 67 % in a certain dose3. In addition, the devices which deliver the drug such as volume spacers have been said to decrease oropharyngeal candidiasis3.
Dry powdered forms of ICS have become very popular in recent years. We investigated Candida colonization and its clinical correlates in asthmatic patients who use fluticasone propionate (FP) as a dry powdered inhaler.
METHODSTwo hundred thirty four asthmatic patients (58 male, 176 female) who were examined in our outpatient department were recruited to the study. 62 patients were taking 200μg/d dry powdered FP and 122 patients were taking 500μg/d dry powdered FP to control symptoms for at least three months. ICSs were delivered twice daily in all patients. 50 asthmatic patients who were seen as a first follow up and diagnosed as asthma were selected as asthmatic controls. They were not taking ICSs at least within the previous three months. 40 normal-nonasthmatic subjects were also used as controls.
None of the subjects were smokers, immunosuppressed, diabetics or had received antibiotic drugs within the previous one month. Patients who were currently taking systemic corticosteroids or had taken them within the previous 6months were not included. In addition to ICSs, all patients with asthma were taking short acting beta-2 agonists as required.
Oropharyngeal fungal cultures were obtained by swabs and kept in a transport medium before being processed in the laboratory where they were cultured on Saboraud's agar. A simple growth on agar slant was considered a positive culture. Candida colonization was defined as positive culture for Candida albicans (C. albicans) and Candida infection was defined as positive oropharyngeal culture for C. albicans along with physical findings and/or symptoms of infection.
Statistical analysisResults are expressed as mean ± SD (standard deviation). Comparisons were done by Mann–Whitney-U test, student t-test and chi-square test when appropriate between groups. We considered a p value less than 0.05 as significant. Multiple logistic regression analysis was used to assess the independent association of potential risk factors on the occurrence of Candida colonization in the patients who were under ICS use.
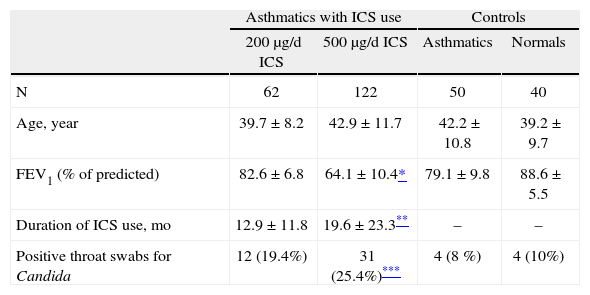
RESULTSDemographic characteristics of the four study groups are shown in table I. Mean ages of subjects in each group were not statistically different (p > 0.05).
Demographic characteristics of the study groups and the frequency rates of Candida colonization
| Asthmatics with ICS use | Controls | |||
| 200μg/d ICS | 500μg/d ICS | Asthmatics | Normals | |
| N | 62 | 122 | 50 | 40 |
| Age, year | 39.7 ± 8.2 | 42.9 ± 11.7 | 42.2 ± 10.8 | 39.2 ± 9.7 |
| FEV1 (% of predicted) | 82.6 ± 6.8 | 64.1 ± 10.4* | 79.1 ± 9.8 | 88.6 ± 5.5 |
| Duration of ICS use, mo | 12.9 ± 11.8 | 19.6 ± 23.3** | – | – |
| Positive throat swabs for Candida | 12 (19.4%) | 31 (25.4%)*** | 4 (8 %) | 4 (10%) |
ICS: inhaled corticosteroids; mo: month.
Table I also shows the frequencies and percentages of positive throat swabs for Candida in the four groups of subjects. Candida colonization was higher in those asthmatics who used 500μg/d FP than controls (table I). Frequency of positive throat swabs for the patients with use of 200μg/d FP was not different from asthmatics without ICS use (x2 = 2.9, p > 0.05) and normal subjects (x2 = 1.7, p > 0.05). There was also no difference between two FP group in this respect (x2 = 1.1, p > 0.05).
We investigated whether asthmatics who were using ICS gargled their throats regularly (after more than 80 % of all uses) or not regularly (gargled after less than 80 % of all uses) within the previous month. 111 patients (60.3 %) reported regular throat wash and 73 (39.7 %) reported irregular throat washing. Patients with regular throat washing had lower Candida colonization than those without regular wash (8 patients, 7.2 % versus 33 patients, 45.2 %; x2 = 37.2, p < 0.0001). There was no difference between 200μg/d FP and 500μg/d FP groups in regard to regular throat washing (x2 = 0.69, p > 0.05). Duration of ICS use was longer in those patients with positive swabs for Candida colonization than those patients with negative culture (22.1 ± 22.6 versus 16.0 ± 19.6months, p < 0.05). Age was higher in patients with positive swabs than patients with negative swabs (44.7 ± 10.6 versus 40.6 ± 9.8years, p < 0.05) Multiple logistic regression analysis was done to determine the possible risk factors (age, ICS dose, duration of ICS use, regular throat wash) which may affect the occurrence of Candida colonization in the asthmatics using ICS. Among them gargling of throat was the most effective variable on the occurrence of colonization (OR: 9.4, 95% CI = 3.9-22.7, p < 0.0001). Use of ICS more than 12months also increased colonization as 2.5 fold when compared with use of less than 12months (OR = 2.5, 95% CI = 1.1-5.6, p < 0.05). Other variables such as older age (OR = 1.03, 95 % CI = 0.9-1.1, p > 0.05) and 500μg/d FP dose (OR = 1.34, 95 % CI = 0.5-3.7, p > 0.05) did not have an important effect on the occurrence of colonization.
DISCUSSIONThe present study showed that ICSs increase oropharyngeal Candida colonization in higher doses of the drug. Although our 200μg/d FP group had a higher frequency of Candida colonization than control groups, it did not reach statistical significance.
Local side effects are mainly seen in patients who use high doses of ICSs. Previous studies confirmed that the incidence of oral candidiasis is related to daily dose of inhaled steroid1,4. Our patients with 500μg FP also showed higher frequency of Candida colonization which is consistent with previous studies. The patients with use of 200μg FP had higher colonization also but it didn't reach statistical significance suggesting that higher daily doses of ICSs cause increased frequency of Candida colonization.
C. albicans, a normal commensal in the mouth, are frequent in patients with some predisposing factors, such as diabetes, immunosuppression or cancers5. Cell mediated immunity plays a major role in the defense against mucocutaneous candidiasis. None of our patients and controls had such predisposing conditions which could affect the frequency of colonization.
The type of ICS has been said to effect the colonization. For example, use of volume spacers reduced oropharyngeal deposition with pMDI than its use alone6. There are limited reports about the effect of dry powdered FP on oropharyngeal Candida colonization. In a previous study, patients with the same doses of pMDI with spacers had a higher frequency of side effects than with Turbuhaler7. Our study adds information about the effect of dry powdered inhalators on oropharyngeal candidiasis in asthmatic patients.
Logistic regression analysis showed that the duration of ICS use was associated with occurrence of Candida colonization. Patients who were users of ICSs for more than 12months had a increased frequency of positive swabs when compared with those patients who had used it for less than 12months which suggested that the duration of ICS use may also affect the colonization in addition to the daily dose of the drug. Although mean ages of patients with positive swabs were higher than patients with negative swabs, it wasn't an important determinant on the occurrence of Candida colonization in multivariate logistic regression analysis.
Oropharnyngeal candidiasis is the most common adverse effect of ICS therapy, but causes no significant morbidity and is easily managed. Our patients with Candida colonization did require to therapy. They were advised to gargle their throats regularly. It's possible to remove 80 % of the dose deposited in the oropharyngeal cavity by rinsing the mouth just once with water. Selroos et al7 also reported that the frequency of Candida colonization in those using dry powdered inhalers was diminished by rinsing the mouth. In another study, clinically significant oropharyngeal candidiasis was reported in about 10 % of adult asthmatics8. Of all patients with positive swabs for Candida, four had clinical thrush and they improved after local antifungal treatment plus discontinuation of the drug.
Gargling the throat was determined as the most effective variable on the occurrence of colonization by multivariate logistic regression analysis in our asthmatic patients. Mouth rinsing after inhalation is recommended by the doctors and the manufacturers, but it's not known how well this is followed by the patients. 60 % of our patients reported to wash their mouth after more than 80 % of all uses. Although we showed that 500μg/day FP dose and longer duration of ICS use cause higher colonization in the asthmatics, regular throat washing was the most effective variable on Candida colonization in the oropharynx. This is important because of the dose and the duration of ICS. It may not be possible to decrease this in certain groups of patients because of their requirements for anti-inflammatory treatment. The deposited portion of the drug in oropharynx can however be removed basically by gargling of the throat.
As a result, regular mouth rinsing should be reminded frequently particularly to those patients who have been using high doses of ICSs and to those who are taking the drug for longer periods of time.