INTRODUCTION
Schnitzler's syndrome (SS) was first reported in 19741. This is an uncommon syndrome, over 50 cases have been reported all over the world and the vast majority come from Europe. It is characterized by chronic urticaria in association with recurrent fever, bone pain and a monoclonal IgM gammopathy1-5. Clinical and laboratory signs of inflammation are also observed. The progress of SS is chronic, but mostly benign. No remissions have yet been reported. A malignant lymphoma may develop in at least 15 % of patients, and in Schnitzler's original patient, Waldenström's disease developed 23 years after SS had appeared6-7. The pathogenesis of the urticaria is unclear and the treatment is difficult8. We report the case of a patient with SS in whom a partial but maintained response after treatment with cyclosporine A (CsA) was observed.
CASE REPORT
A 61-year-old woman had longstanding (4-years) urticaria, associated with recurrent fever and arthralgias. In the last year pruritus and skin lesions became resistant to antihistamines and oral steroids. Urticarial lesions appeared daily. Recurrent febrile episodes were up to 39 °C and self-limited. Deterioration of his general condition was observed. No episodes of angioedema were observed. The patient had had 3 episodes of bacterial pneumonia. Physical examination revealed numerous urticarial plaques (between 1 and 10 cm) on her trunk and limbs, and in the proximal part of legs and arms. The remainder of the physical examination was normal (no hepatomegaly, splenomegaly or lymphoadenopathy). Histology of skin revealed edema of the superficial dermis and moderate mononuclear and polymorfonuclear cell infiltrate around superficial blood vessels. Leucocytoclastic vasculitis was also observed. Bone marrow aspirate and biopsy did not reveal lymphoplasmocitoid proliferation. Studies to detect mastocytosis were also negative (tryptase, 5-hidroxy-indolacetic acid and histamine levels were normal). A CT-scan of thorax and abdomen was normal. Gynaecological exploration was normal. Laboratory investigations performed at different times of follow-up revealed: a rise in erythrocyte sedimentation rate (> 50 mm/h), increased C-reactive protein (> 9 mg/dL), high level of IgM (> 500 mg/dl), whereas IgG, IgA and IgE levels were normal. Decreased levels of IgG1 (475 mg/dL), IgG4 (6 mg/dL) and of the ability to produce antibodies to tetanus toxoid after vaccination were observed. C4 levels were low (< 16 mg/dL). Electrophoresis and immunoelectrophoresis provided evidence for the existence of underlying serum IgM kappa paraprotein. Kappa/Lamda ratio was increased (5.28). Urinary Bence-Jones protein was absent; beta-2-microglobulin was normal; no hypercalcemia was observed. There were no cryoglobulins, no cryoagglutinins, rheumatoid factor, antinuclear antibodies or anti-thyroid antibodies. C3 and C1 esterase-inhibitor (C1-inh) levels and C1-inh function were normal. T and B lymphocyte subsets were normal: CD3: 77 % (1771 cells/mL), CD4: 46 % (1058 cells/mL), CD8: 30 % (690/mL), CD56: 13 % (299 cells/mL), CD19: 8 % (184 cells/mL). Radiograph studies disclosed no osteolytic lesions. Serology to EBV (IgG), cytomegalovirus (IgM), micoplasma (IgG), hepatitis virus B, hepatitis virus C and echinococcus granulosus was negative. T4 thyroid hormone and TSH levels were normal. Serial stool samples disclosed no parasites.
Treatment protocol: Because of recurrent febril episodes, malaise along with clinical signs of leucocitoclastic vasculitis without response to corticosteroids, she received low-dose cyclosporine (CsA) (2,5 mg/kg per day for 16 weeks) on a compassionate basis in combination with cetirizine (10 mg/day) and then she was followed up for 18 months. The CsA dosages were adjusted to a target range of 100-200 ng/mL during the treatment period.
A clinical assessment, blood count and biochemical profile were done at weeks 0, 1 and reviewed at 2-week intervals for two months, then monthly.
Weekly aggregate urticaria activity score (UAS) was registered. Daily record for the preceding 24 h of small (< 3 cm) and large (> 3 cm) weal numbers, scored as follows: 0, < 10 small weals; 1, 10-50 small weals or < 10 large weals; 2, > 50 small weals or 10-50 large weals; 3 almost covered. Severity of itch was scored as 0, none; 1, mild; 2, moderate; 3, severe.
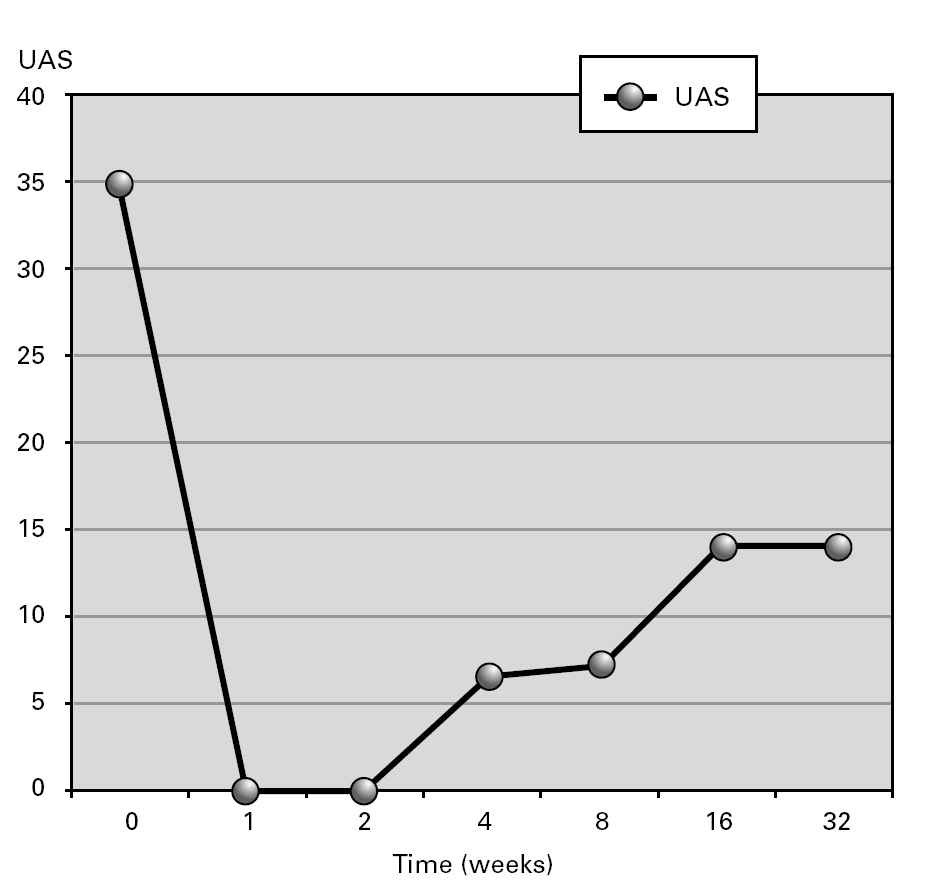
Outcome: After 4 years of therapeutic failure, CsA achieved a partial clearance of urticarial lesions (fig. 1). Treatment was well tolerated. The patient's condition improved, allowing decrease in corticosteroid doses. Eighteen months later, except for urticaria, clinical features (recurrent fever, arthralgias and malaise) have disappeared. Urticaria relapses resolved spontaneously or with low dose steroids (6 mg methylprednisolone each 48 hours). During treatment with CsA a transitory increase of IgM was observed (615 to 1550 mg/dl) which reached baseline levels after CsA was finished. No hematological disorder has been observed during post-treatment follow-up. The paraprotein remain stable.
Figure 1.--Aggregate urticaria activity score (UAS) during follow-up in a patient with Schnitzler's Syndrome during treatment with Cyclosporine.
DISCUSSION
Paraproteins represent a condition characterized by clonal proliferation of immunoglobulin producing plasma-B-cells. Skin disorders may be associated with paraproteins. These skin disorders can occurr by colonization of the plasma cell clone in the dermis expressed as a deposition of proteins, such as in the case of AL amyloidosis and cryoglobulinemia. SS is another type of syndrome that is highly associated with an M component1. The M component use to be of the IgM-kappa type. It is not yet firmly established whether the monoclonal immunoglobulin component plays a part in the pathophysiology of the urticarial lesions. Lipsker et al described the presence of anti-skin IgM autoantibodies of the same isotype as their monoclonal gammopathies in the serum of some patients with the SS8-9.
The SS is a probable underdiagnosed systemic urticarial eruption in patients with a monoclonal IgM gammopathy1-5. Mean age at disease onset is 60 years and, in most cases, the delay to diagnosis is longer than 5 years. IgM paraprotein levels can remain stable or increase progressively1-5. In our patient an increase in IgM serum concentration was observed before and during treatment with CsA which return to baseline levels after treatment was completed. SS has an important percentage of evolution to lymphoproliferative malignancy in the course of the time, making the diagnosis and a careful follow-up necessary6-7.
Treatment of SS is difficult and unsatisfactory. No treatment is constantly effective against the skin rash. Ignoring the exact mechanisms of the pathogenesis of SS, many drugs have been tried. Antihistamines are ineffective. Nonsteroidal anti-inflammatory drugs, dapsone, chloroquine, colchicine and immuno-supppresive treatments have been tried in different patients with contradictory and often disappointing results10,11. Systemic corticosteroids, alone or in combination, are sometimes helpful but high doses and long-term treatment are generally necessary.
CsA has been used for the treatment of refractory idiopathic urticaria12-13. CsA in addition to background treatment with cetirizine has proved useful in the treatment of chronic idiopathic urticaria in a recent double-blind, randomized clinical trial14. Pascual-López et al reported on a patient with SS who had leucocytoclastic vasculitis. After 20 years of therapeutic failure, a total clearance of urticarial lesions was obtained with CsA15. Low dose CsA proved efficacious in our patient with SS. Disabling skin rash, fever, malaise and musculoskeletal involvement improved, leading to a better quality of life of the patient. Urticaria relapses after treatment with CsA were easily controlled with antihistamines and low-dose corticosteroids. Control of febrile episodes with continuing skin involvement has been reported in other cases.
Correspondence:
Dr. Javier Carbone
Clinical Immunology Unit.
University Hospital Gregorio Marañón,
Dr. Esquerdo, 46, 28007 Madrid, Spain.
Phone: 34 91 4265180. Fax: 34 91 5866698
E-mail: carbone@teleline.es