INTRODUCTION
Asthma complicates the course of 200,000 to > 300,000 pregnancies every year1, and approximately 4 % to 7 % of pregnant women receive drug therapy for asthma2. Studies including information from a large number of women exposed to inhaled corticosteroids showed no increased risk of adverse maternal or fetal outcomes3,4. Although it is very unlikely that pregnancy outcome following exposure to this specific inhaled corticosteroid may differ from the results found in studies evaluating the inhaled corticosteroids as a group, there was no yet specific information on fluticasone for counseling exposed pregnant women.
MATERIAL AND METHODS
The study was approved by the Ethics and Research Committees at the Cheil Hospital and Women's Health Care Centre. The cases were identified at the Korean Motherisk Program and all participants provided written informed consent to participate in the study.
A detailed medical and obstetric history was obtained from participants who were using fluticasone during pregnancy. Since there was no specific information about fluticasone, we provide them with oral and, if requested, written teratogen-risk counseling based on the extensive information available on inhaled corticosteroids as a group. If participants were also exposed to other medications, the concomitant treatment was also discussed during the teratogen-risk counseling.
Cases were periodically followed-up at the hospital and if required, they underwent blood tests, ultrasound obstetric examinations, and amniocentesis. In addition, we prescribed any treatment that participants might have required during pregnancy. However, the physician taking care of the patients' respiratory illness was responsible of any change in their prescription related to the inhaled corticosteroids.
Follow-up was performed until participants had spontaneous abortion, underwent voluntary pregnancy termination, or delivery. If delivery occurred, the baby was carefully examined by a pediatrician in order to intentionally rule out any gross malformation or neurodevelopmental delay at birth. The pediatrician was not aware of the mothers' drug therapy during pregnancy.
RESULTS
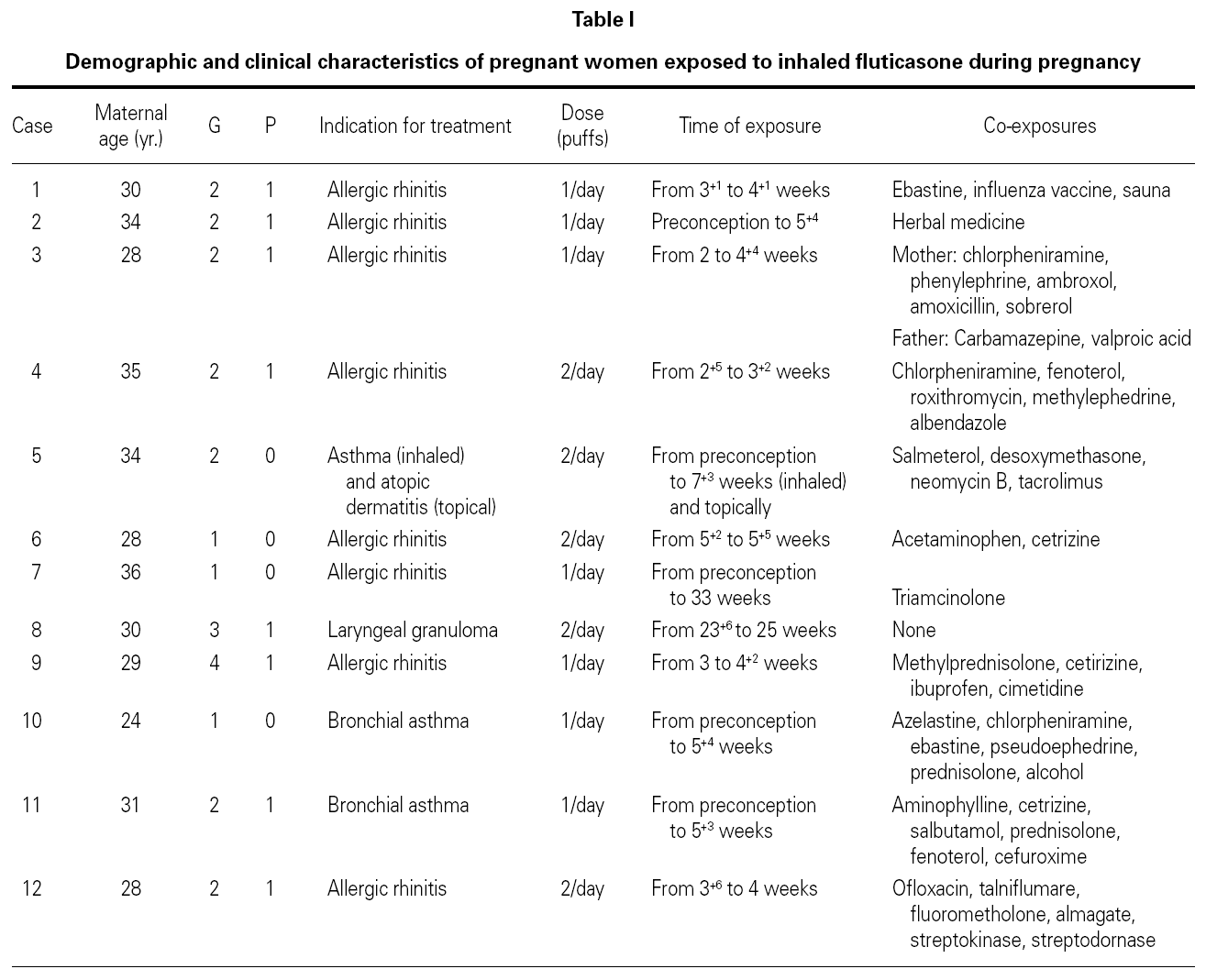
We identified 12 pregnant women with respiratory illnesses who were treated during pregnancy with inhaled fluticasone (Table I). However, most of them were also exposed to at least one of the following medications: antacid compounds (almagate), anthelmintics (albendazole), antibiotics (cefuroxime, ofloxacin, roxithromycin), anti-inflammatory molecules with mucoregulator properties (talniflumate), expectorants (ambroxol, sobrerol), fibrinolytics (streptokinase-streptodornase), inhaled bronchodilators (aminophylline, fenoterol, salbutamol), inhaled histamine H1 receptor antagonists (azelastine), non-steroidal anti-inflammatory drugs (acetaminophen, ibuprofen), ophthalmic corticosteroids (fluorometholone), oral corticosteroids (desoxymethasone, methylprednisolone, prednisolone), oral histamine H1 receptor antagonists (chlorpheniramine, cetirizine, ebastine), oral histamine H2 receptor antagonists (cimetidine), oral sympathomimetic amines (methylephedrine, phenylephrine), and topical corticosteroids (triamcinolone).
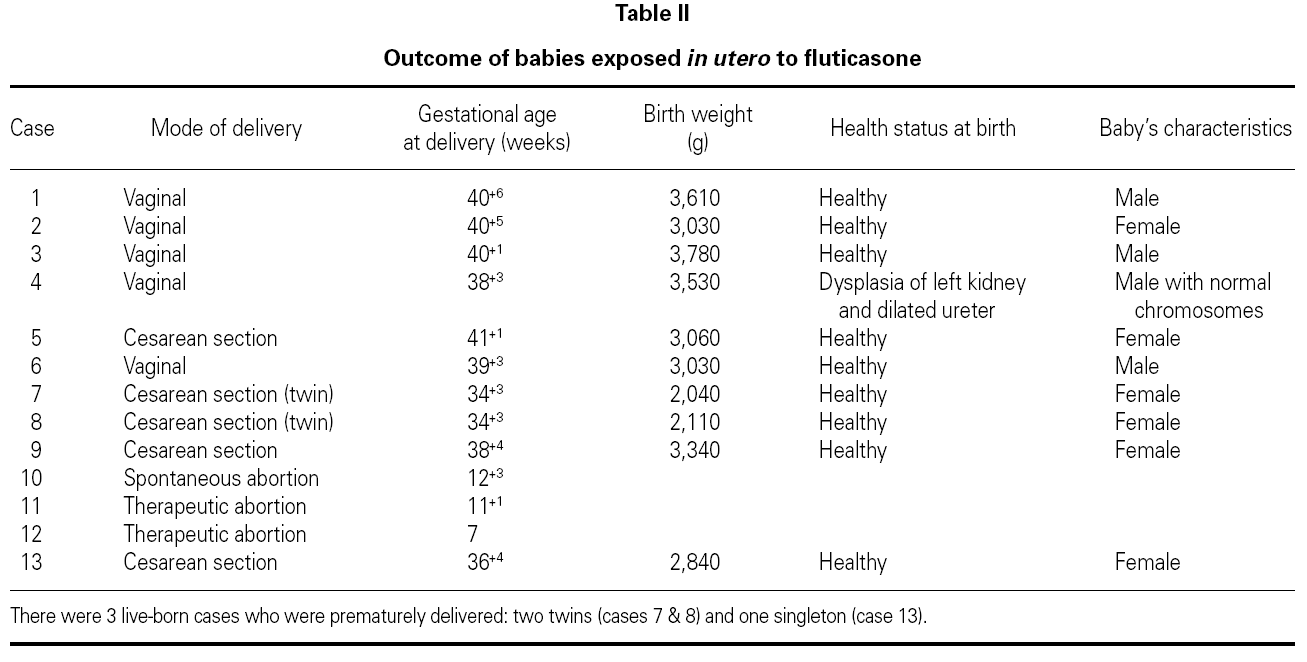
During pregnancy, none of the 12 women exposed to inhaled fluticasone moved out of control of their respiratory illness or had any complication including hypertension, diabetes, or eclampsia. There were abortions: one spontaneous and 2 requested by the patients arguing personal reasons. In total, 10 babies were delivered: 8 singleton babies and 2 twins; the twins were born at 34+3 weeks and one of the singletons was born at 36+4 weeks (Table II). No evidence of major congenital malformations or neurodevelopmental delay was observed in any baby at birth or at their first postnatal visit 1 week later.
DISCUSSION
Despite the extensive information on the safety use of inhaled corticosteroids during pregnancy, this appears to be the first report specifically focused on the lack of adverse maternal and fetal outcomes of fluticasone when used during pregnancy. This inhaled corticosteroid has high receptor-binding affinities, enhanced respiratory effects through prolonged pulmonary residence time, limited systemic effects through negligible oral bioavailability (less than 1 %), and rapid hepatic clearance5. Fluticasone has effectively been used for the treatment of allergic rhinitis and acute and chronic treatment of asthma in children and adults. At established therapeutic dose-response range of 50 to 500 mg/day, fluticasone has minimal effects on adrenal function in asthmatic adults6.
Several cohort studies have shown that appropriate use of inhaled corticosteroids and beta-agonists do not add any risk to pregnant women or the developing fetus3,4,7-9. In addition, controlled asthma is associated with improved pregnancy outcomes. The guidelines on asthma during pregnancy by the National Heart, Lung, and Blood Institute emphasize continuing treatment of asthma throughout pregnancy10. Despite these encouraging results and the mentioned guidelines, a recent study documented a drop of 23 % in the use of inhaled corticosteroids, 13 % of beta-agonists, and 54 % of rescue corticosteroids among women in the first trimester of pregnancy11. It is probably that the negative perceptions on the use of corticosteroids in pregnancy is based on some studies showing that the use of oral corticosteroids in pregnancy, not inhaled therapy, increase the risk of oral clefts. A relatively increased risk has also been reported for topical corticosteroids12,13. However, the increased risk with systemic corticosteroids appears to be marginal and has not been consistently observed across all the published epidemiological studies14-16. It is therefore unlikely that corticosteroids administered by other routes with substantially low systemic bioavailability may increase the risk of adverse pregnancy outcomes. In this context, our case series on inhaled fluticasone in pregnancy can be used to emphasize the safety of inhaled corticosteroids in pregnancy.
In conclusion, inhaled fluticasone was not associated with adverse maternal or fetal outcomes. Although our study is limited to a case series of 10 live born babies, our results are in agreement with previous studies where no increased rate of adverse outcomes were reported with the use of inhaled corticosteroids during pregnancy.
Correspondence:
Jung Yeol Han, MD
The Korean Motherisk Program
Department of Obstetrics and Gynecology
Cheil General Hospital & Women's Healthcare Center
1-19 Mookjung Dong, Choong Gu
Seoul, Korea 100-380
E-mail: hanjungyeol@yahoo.com