INTRODUCTION
In the last years the Cupressaceae family plants are undergoing a strong geographical expansion, due to their use as physical barrier or as ornamental motives. Therefore, Cupressaceae pollens are an important cause of pollen allergy in several regions of the world. In Mediterranean countries the prevalence to Cupressaceae pollens can reach until 30 % of the pollinosis cases1-3.
Extensive cross-reactivity of the allergens has been demonstrated between different Cupressus and Juniperus species4,5. This fact together with the overlapping of their pollinitation periods make that the presence of allergic symptoms was continuous from October to April. Cupressaceae allergic patients present different clinical characteristics to other pollinosis: the dominant symptom is rhinitis occasionally associated to conjunctivitis, while the asthma incidence is minor than in other pollen allergies; the patients usually have low levels of specific IgE and they develop symptoms older than in other pollinosis6,7. As a consequence of these low levels of specific IgE in patient sera, the in vitro inhibition studies used at this moment for the standardization of allergenic extracts are difficult to carry out. Moreover, Cupressaceae family pollen extracts are characterized to have a high content in carbohydrates and a low protein content (3 %)4, what has hindered the possibility of producing good standardized extracts for diagnosis and immunotherapy.
Allergen content can be quantified in mass units by using monoclonal (mAb) and/or polyclonal antibodies specific for the allergen. The Cupressus sempervirens major allergen is a 43 kDa protein, named Cup s 18 that has been recently purified9 and cloned (EBI accession number AF7257491), sharing a high homology with Cup a 1. Recently, a mAb-based ELISA has been developed in our lab for quantification of Cup a 110, but no accurate determination can be obtained for C. sempervirens and other Cupressaceae species. In this work we describe a two-site sandwich ELISA for the quantification of Cup s 1 in C. sempervirens pollen extracts, using a monoclonal antibody as capture and a monospecific polyclonal antibodies as detector.
MATERIALS AND METHODS
Allergenic extracts and Cup s 1 purification
In the preparation of the allergenic extracts, pollen of C. sempervirens, C. arizonica, Thuja plicata, and Juniperus communis (Iber-Polen, Málaga, Spain) were defatted and extracted during 4 h at 4 °C in 50 mM phosphate buffer pH 8.0 containing 65 mM NaCl. The obtained extracts were clarified by centrifugation to 5000 xg for 30 min, filtrated through AP glass fiber prefiltrer (Millipore Corp., Bedford, Madison, USA) and dialyzed by ultrafiltration with a 5000 Daltons exclusion size in a Pellicon System (Millipore). In order to improve Cup s 1 purification, C. sempervirens pollen was washed for 10 min in 10 mM KH3PO4 pH 4.3, followed by an extraction with 40 mM NH4HCO3 at 4 °C11. 2 M (NH4)2SO4 was added to the clarified supernatant and was applied to a High Flow Phenyl-Sepharose 16/20 column equilibrated with 20 mM phosphate buffer pH 7.0, containing 2 M (NH4)2SO4 in the ÄKTA-prime SystemTM (Amersham Biosciences, Buckinghamsire, UK). The bound fraction was eluted with 20 mM phosphate buffer pH 7.0, concentrated and applied to a Superdex S-200 16/60 column equilibrated with phosphate buffer saline (PBS). The fraction containing the 43 kDa protein was collected, and after a dialysis with 50 mM sodium acetate pH 5.0, it was injected in a Mono S 1.6/5 ion exchange column into a Smart SystemTM (Amersham Biosciences). The purified allergen was eluted as a unique peak at 100 mM NaCl. The protein content was determined by the Bradford method with the Bio-Rad protein assay kit (Bio-Rad, Hercules, California, USA). Carbohydrate content was measured as previously reported12.
Analysis of the pectate lyase enzymatic activity
The pectate lyase activity was analyzed according Keen et al13. Briefly, the reaction mixture contained 0-0.2 % (w/v) of lemon pectin (Poly-D-galacturonic acid methyl ester, Fluka, Bush, Switzerland), 50 mM Tris (pH 8.5), 5 mM CaCl2 and 5 mg of purified Cup s 1 in 1 ml of total volume. The reaction was carried out at 37 °C for 1 hour, and the absorbance was measured at 235 nm. An unit of pectate lyase activity is defined as the amount needed to form 1 mM of product (unsaturated uronides) per minute with a molar extinction coefficient of 4600 M1 at 235 nm.
Production, purification and biotinylation of antibodies
The production and characterization of mAb 3D2, recognizing the major allergen of different Cupressaceae, has already been described previously10. Polyclonal antibodies were obtained in New Zealand rabbits after 5 boosts of 200 mg of the purified Cup s 1 every 2 weeks, emulsified in Freund's complete adjuvant (Difco Laboratories, Detroit, Michigan, USA). Both, monoclonal and polyclonal antibodies were purified by affinity chromatography in HiTrap Protein G columns (Amersham Biosciences). The anti-Cup s 1 rabbit purified immunoglobulins were marked with biotin by the ECL protein biotinylation moduleTM (Amersham Biosciences).
Electrophoresis and Immunoblotting
The protein bands of the pollen extracts and of Cup s 1 were detected with Coomassie brilliant blue after being separated by SDS-PAGE at 12.5 % acrylamide under reducing conditions according to Laemmli method14. For the immunodetection assays, the proteins were transferred to Hybond-P membranes (Amersham Biosciences)15 and blocked for 1 h at room temperature with PBS containing 0.1 % Tween-20. Next, the membranes were incubated under similar conditions with mAb 3D2 (0.5 mg/ml) or with anti-Cup s 1 rabbit antiserum (diluted 1:500,000). After washing, blots were incubated with the corresponding peroxidase-labeled secondary antibody and developed by the ECL western blotting detection system (Amersham Biosciences).
Quantification of Cup s 1 by a two-site sandwich ELISA
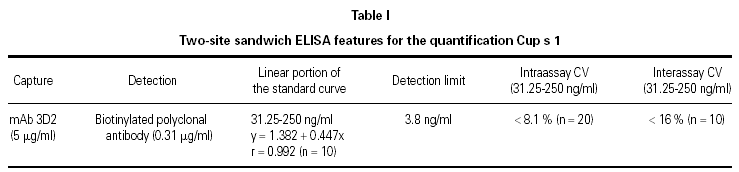
Microwell plates (Greiner, Frickenhausen, Germany) were coated overnight at room temperature with 100 ml/well of the mAb 3D2 at 5 mg/ml in PBS. Next, plates were blocked by adding 200 ml/well of PBS-1 % BSA-0.05 % Tween 20 (PBS-B-T) and incubated 1 h at 37 °C. Afterwards, wells were incubated with 100 ml/well of purified Cup s 1 (2000-2 ng/ml), or with the C. sempervirens pollen extracts (10000-25 ng/ml) in PBS-B-T, followed by a second incubation with biotin-labeled anti-Cup s 1 rabbit polyclonal antibody (0.31 mg/ml) and finally with streptavidin-peroxidase (0.25 mg/ml) (Sigma). All the incubations were carried out at 37 °C during 1 h, and with 3 washes of 200 ml/well of PBS-T between incubations. Color development was performed at room temperature and in darkness with a solution of o-phenylenediamine (Sigma-Fast o-phenylenediamine dihidrochloride Tablet Sets, Sigma). The reaction was stopped at 30 min with 50 ml/well of 3 M H2SO4, and the optical density was measured at 492 nm. Detection limit was calculated as the amount of Cup s 1 which corresponded to the mean plus 3.33 times the standard deviation obtained after 30 measurements of the zero standard. Intraassay variation coefficients were calculated measuring the absorbance of 20 wells at concentrations of 31.25-62.5-125-250 ng/ml. Interassay variation coefficients were determined in 10 standard curves carried out with Cup s 1 in different days. The extracts were assayed in triplicate and the concentrations were interpolated in the lineal portion of the standard curve.
RESULTS
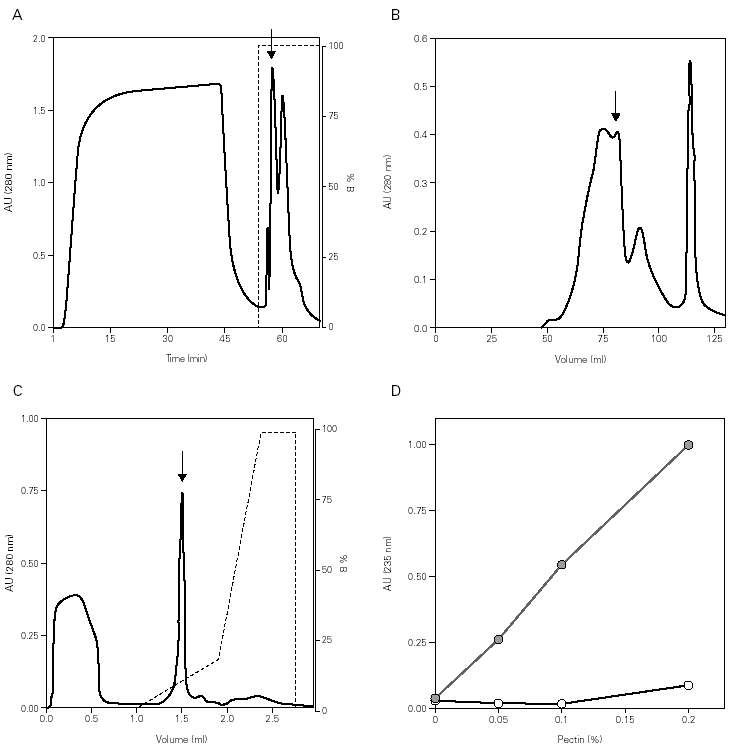
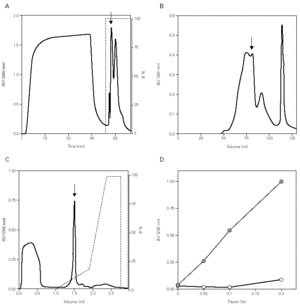
In the Cup s 1 purification, the pollen was subjected to a quick wash with low ionic content and acidic pH buffer, followed by an extraction at basic pH with the purpose of eliminating most of the non-protein components. The obtained supernatant was supplemented with 2 M (NH4)2SO4 with the purpose of promoting the protein binding to phenyl-sepharose resin. After washing, the bound fraction was eluted with phosphate buffer, and contained a high concentration of proteins with low amounts of carbohydrates (fig. 1A). Further purification was obtained by gel filtration chromatography where Cup s 1 was contained in the 40-50 kDa peak (fig. 1B). This fraction was subjected to a last purification step in a Smart System using a cation exchange column. Cup s 1 was eluted with 100 mM NaCl as a 43 kDa band while the rest of proteins come out in the non-bound fraction at pH 5.0 (fig. 1C). The purification yield was 1.5 % of the total extract proteins.
Figure 1.--Purification of Cup s 1 from C. sempervirens extracts. Elution profiles after: (A) Phenyl-Sepharose interaction hydrophobic chromatography. (B) S-200 gel permeation chromatography. (C) Mono-S ion exchange chromatography. Arrows indicate Cup s 1 fractions. (D) Pectate lyase activity of Cup s 1 to different pectin concentrations in absence (s) and in presence (•) of calcium.
The identification of the purified protein as Cup s 1 was proven by immunoblotting with specific antibodies, as well as for its pectate lyase activity. Cup s 1 possesses the capacity to degrade pectin with a specific activity of 725 U/mg protein, and this activity is also completely dependent of calcium (fig. 1D). This is the first time it has been demonstrated that Cup s 1 is a functional active pectate lyase enzyme.
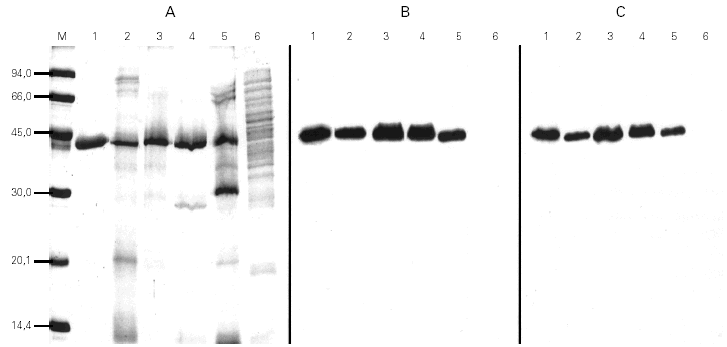
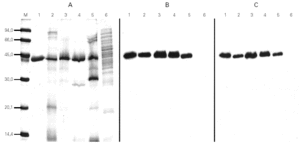
For the quantification of Cup s 1 in the two-site sandwich ELISA, the mAb 3D2 was used as capture and the biotin-labeled anti-Cup a 1 polyclonal antibody as detector. Both were specific to a 43 kDa protein presented in pollen extracts from Cupressaceae family (Cupressus, Thuja, Juniperus), but these antibodies did not react with proteins from other taxonomically related pollen extracts (Pinus and Cedrus) (fig. 2). Purified Cup s 1 was used as reference material in a standard curve with concentrations between 2000 and 2 ng/ml. The working range of the assay was defined as the lineal portion of the curve, between 250 and 31.25 ng/ml. The detection limit was calculated as 3.8 ng/ml. The assay was reproducible, since the intraassay variation coefficients at 250, 125, 62.5, and 31.25 ng/ml were of 7.6, 7.0, 8.1, and 6.7 %, respectively. The interassay variation coefficients for the same points were always minor than 16 % (table I).
Figure 2.--Coomassie stained SDS-PAGE (A) and immunoblots incubated with mAb 3D2 (B) or the polyclonal antibody (C) of purified Cup s 1 (lane 1), of pollen extracts from C. sempervirens (lane 2), C. arizonica (lane 3), T. plicata (lane 4), J. communis (lane 5) and P. radiata (lane 6).
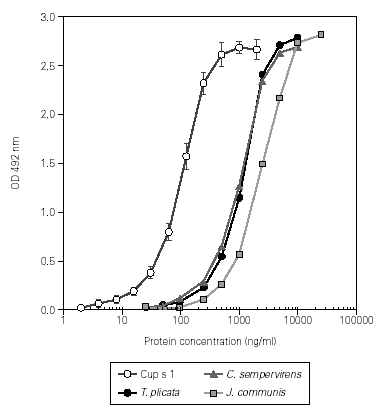
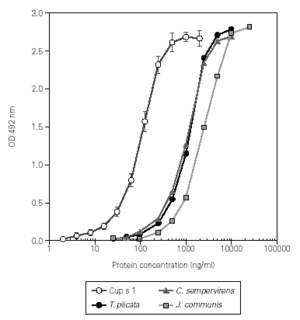
This assay was used to quantify the Cup s 1 content in different batches of C. sempervirens pollen extracts, ranging from 73 to 95 mg/mg de protein. The extract dose-response curves showed a good parallelism when compared with the obtained with the purified allergen, indicating that the same protein was measured (fig. 3). The Cup s 1 standard curve was applied to the quantification of Cup s 1-like proteins in T. plicata and J. communis pollen extracts, with an allergen content of 75 and 50 mg/mg de protein, respectively. Nevertheless, for accurate quantification, the corresponding purified group 1 allergen for each extract should be used as reference in the dose-response curve. The specificity of the assay was tested using extracts of non-plant origin (Alternaria alternata and Dermatophagoides pteronyssinus), and pollen extracts from Olea europaea, Platanus acerifolia, Betula verrucosa, Pinus radiata, Cedrus atlantica, Dactylis glomerata, Helianthus annuus, Parietaria judaica, Artemisia vulgaris and Plantago lanceolata. No reactivity was detected between 0.25 and 25 mg/ml of protein in any case.
Figure 3.--Dose-response curves in ELISA of purified Cup s 1, and the pollen extracts from C. sempervirens, T. plicata and J. communis.
DISCUSSION
The cypress pollen extracts are characterized by their high carbohydrate and low protein content, due to the physical-chemical properties of the pollen grains6. Efforts have been made to establish the optimal conditions for pollen extraction and to provide qualitative definition of the relevant allergenic components in these extracts4,8,11,16,17.
The C. sempervirens major allergen purification was carried out by the same method than the one described for Cup a 110,11, but including an additional step based on a cation exchange chromatography at pH 5.0. This step purified the protein from the low molecular weight proteins present in the C. sempervirens extract. Cup s 1 had more than 98 % purity and was used as standard in the two-site sandwich ELISA. The allergen had high homology with other cypress group 1 allergens (Cry j 1, Jun a 1, Cha o 1, Cup a 1) ranging from 80 % identical amino acids with Cry j 1 to 96 % with Jun a 1. Cypress group 1 allergens have been described as enzymes with pectate lyase activity dependent of Ca+2 ion, but functional enzymatic activity have been only demonstrated for Cry j 1 and Cup a 110,18. Here, we have shown that purified Cup s 1 has pectate lyase activity with a specific activity of 725 U/mg protein, comparable to ones described for Cup a 1 (1200 U/mg) and Cry j 1 (350 U/mg)10,18.
The allergenic extract standardization is usually carried out by means of in vitro techniques using allergic patient sera, which are difficult to homogenize. In the case of Cupressaceae allergy, the finding of good sera become more difficult since these patients have low levels of specific IgE to cypress extracts6. Therefore, the development of an immunoassay for the quantification of Cup s 1 is of great interest as a complementary tool for standardization. The success in the development of this type of procedures is closely linked to the existence of specific antibodies and the availability of a high grade purity standard.
The mAb 3D2, used as capture mAb, reacted with Cup s 1, Cup a 1 and a corresponding protein of T. plicata and J. communis pollen extracts. Therefore, it seems to detect a conserved epitope presented in Cupressaceae proteins and becomes a useful tool in the immunoassays for the quantification of major allergen within the Cupressaceae family. Nevertheless, the corresponding purified allergen for each extract should be used for an accurate quantification of the allergen content expressed in mass units. The use of polyclonal antibodies as tracer did not diminish the assay specificity, since this second antibody did not show reaction to any other proteins, even from Pinus or Cedrus extracts, plants from the Pinaceae family included, as the Cupressaceae family, inside the Coniferal order. Using the described assay, allergen concentration of 7.3, 7.5 and 5 % for pollen extracts of C. sempervirens, T. plicata and J. communis pollen seems to be more compatible with data of protein yield after purification and immunoblotting than allergen concentration of 0.5, 1.2, and 1.6 % obtained previously using a sandwich ELISA based on two mAbs10.
The ELISA could be applicable to the routine analyses of cypress extracts used to specific immunotherapy and diagnostic, given the high sensitivity of the assay. The reproducibility, just like the sensitivity and specificity requirements, was also fulfilled in the two-site sandwich ELISA, with acceptable intraassay and interassay variation coefficients.
In conclusion, this paper describes a two-site sandwich ELISA procedure for measurement of Cup s 1 in C. sempervirens pollen extracts. This assay results useful due to its specificity, sensitivity and reproducibility, and it can be applicable to the quantification of the other Cupressaceae major allergens.
ACKNOWLEDGMENTS
This work was supported in part by Bial-Arístegui and by Grants FIT-090000-2003-61 from the Plan Nacional de I + D (Programa PROFIT, Ministerio de Ciencia y Tecnología, Spain), and TEI-0163-2002 from the Programa INTEK (Departamento de Industria, Agricultura y Pesca, Gobierno Vasco).