Tuberculosis is a curable infectious disease, prevalent worldwide and potentially fatal if proper treatment is not instituted on time. Currently the therapeutic regimens include isoniazid (INH), rifampicin (RIF), pyrazinamide (PZA), ethambutol (EMB) and streptomycin, all associated to a high rate of adverse effects which can lead to treatment failure.1 Hypersensitivity reactions to antituberculosis are seen in 4–5% of the general population.2 In cases of such vital indication, when there is no alternative treatment available, drug desensitisation may be indicated. However, it should only be performed by specialised and experienced physicians and under intensive surveillance.3
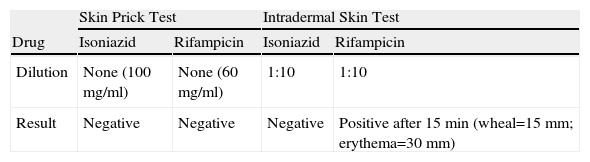
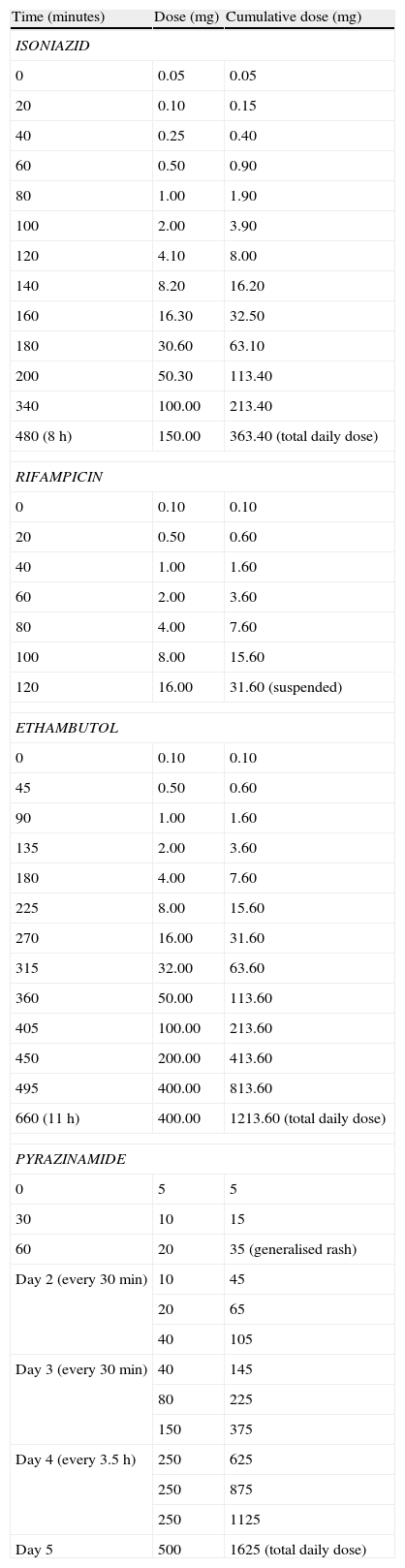
We report a case of a 26-year-old man, smoker, with no previous pathologic condition, who was admitted to an Infectiology Unit with the diagnosis of pulmonary tuberculosis and started on a regimen of INH, RIF, PZA and EMB at the recommended dosages. On the tenth day of therapy he developed a generalised pruriginous maculopapular rash, fever and diarrhoea which progressively disappeared in the following days. Four days later, all drugs except RIF where reintroduced in the same posological regimen and 1.5h later he developed fever, hypotension, generalised maculopapular rash, as well as eosinophilia and rise in hepatic transaminases, with progressive regression of the symptoms. A third attempt at treatment was made 3 days later, with RIF and EMB, with the development of dyspnoea, hypotension, hypoxaemia, and generalised maculopapular rash 2.5h later. Once there was complete regression of symptoms, INH was administered, with the development of fever and generalised maculopapular rash 10h later. After regression of symptoms, PZA was administered, with the development of fever, generalised maculopapular rash and redness and oedema of the conjunctiva 45min later. All the reactions led to suspension of the drugs until complete remission of symptoms. The severe reactions were treated with adrenaline, corticotherapy, hydroxizine and supplemental oxigenotherapy and the milder reactions with hydroxizine. Given the need of rapid re-institution of therapy, our Immunoallergy Department was contacted for evaluation. Skin testing was performed to INH and RIF, using the pure substance to the prick test and a 1:10 dilution to the intradermal test (Table 1). The RIF intradermal reaction was positive after 15min, with a wheal of 15mm and an erythema of 30mm. Rapid oral tolerance induction protocols were planned based on recent published literature. Informed consent was obtained and all the procedures were done in the Infectiology Unit with resuscitation equipment at bedside. All protocols were implemented assuring tolerance to the therapeutic dosage of each drug before advancing to the next (Table 2). The first protocol applied concerned INH. Given the severity of the reaction, we started with 0.01% of the total daily dosage, taking 8h to reach the daily dose, with no intercurrences. The second protocol was with RIF, also starting with 0.01% of the total daily dosage. The patient developed a generalised rash with a cumulative dosage of 31.6mg (2h after the first administration), easily controlled with hydroxizine. However, the Infectiology Unit's medical staff decided to suspend the protocol and advance to the next – EMB. As with the previous protocols, we started with 0.01% of the total daily dosage (1200mg), and 11h later reached the daily dose, with no symptoms. The last protocol applied concerned PZA. Starting with 0.3% of the total daily dosage (1500mg), the patient developed a generalised rash with a cumulative dosage of 35mg (1h after the first administration), easily controlled with hydroxizine. Once there was complete remission of the symptoms, the last tolerated dose was administered and the protocol was completed with some adjustments, taking 5 days to reach the daily dose. The patient has completed the triple treatment (INH, EMB, PZA) asymptomatically. Blood tests were regularly performed in order to monitor eventual alterations: all the previously altered parameters progressively returned to normal and there weren’t further changes.
Rapid oral tolerance induction protocols
| Time (minutes) | Dose (mg) | Cumulative dose (mg) |
| ISONIAZID | ||
| 0 | 0.05 | 0.05 |
| 20 | 0.10 | 0.15 |
| 40 | 0.25 | 0.40 |
| 60 | 0.50 | 0.90 |
| 80 | 1.00 | 1.90 |
| 100 | 2.00 | 3.90 |
| 120 | 4.10 | 8.00 |
| 140 | 8.20 | 16.20 |
| 160 | 16.30 | 32.50 |
| 180 | 30.60 | 63.10 |
| 200 | 50.30 | 113.40 |
| 340 | 100.00 | 213.40 |
| 480 (8h) | 150.00 | 363.40 (total daily dose) |
| RIFAMPICIN | ||
| 0 | 0.10 | 0.10 |
| 20 | 0.50 | 0.60 |
| 40 | 1.00 | 1.60 |
| 60 | 2.00 | 3.60 |
| 80 | 4.00 | 7.60 |
| 100 | 8.00 | 15.60 |
| 120 | 16.00 | 31.60 (suspended) |
| ETHAMBUTOL | ||
| 0 | 0.10 | 0.10 |
| 45 | 0.50 | 0.60 |
| 90 | 1.00 | 1.60 |
| 135 | 2.00 | 3.60 |
| 180 | 4.00 | 7.60 |
| 225 | 8.00 | 15.60 |
| 270 | 16.00 | 31.60 |
| 315 | 32.00 | 63.60 |
| 360 | 50.00 | 113.60 |
| 405 | 100.00 | 213.60 |
| 450 | 200.00 | 413.60 |
| 495 | 400.00 | 813.60 |
| 660 (11h) | 400.00 | 1213.60 (total daily dose) |
| PYRAZINAMIDE | ||
| 0 | 5 | 5 |
| 30 | 10 | 15 |
| 60 | 20 | 35 (generalised rash) |
| Day 2 (every 30min) | 10 | 45 |
| 20 | 65 | |
| 40 | 105 | |
| Day 3 (every 30min) | 40 | 145 |
| 80 | 225 | |
| 150 | 375 | |
| Day 4 (every 3.5h) | 250 | 625 |
| 250 | 875 | |
| 250 | 1125 | |
| Day 5 | 500 | 1625 (total daily dose) |
Adverse reactions to antituberculosis agents, in particular hypersensitivity reactions, have important diagnostic and therapeutic limitations for several reasons: (i) the mechanisms responsible for the hypersensitivity reactions are still not completely understood for the majority of these drugs; (ii) diagnostic tests for immunological confirmation have been rarely evaluated; (iii) little is known about the mechanism of drug desensitisation.1–4
As far as we know this patient is the first reported case of hypersensitivity to INH, RIF and PZA with successful rapid oral tolerance induction to INH and PZA.
Adverse drug reactions (ADR) to INH occur in about 5% of patients.1,4 The most common ADR involve hepatic cytolysis, peripheral neuropathy, skin rashes (morbilliform, maculopapular or urticarial rash, contact dermatitis), lung infiltrates, interstitial pneumonia, leucopoenia, DRESS syndrome (Drug Rash with Eosinophilia and Systemic Symptoms), isolated fever, flu-like syndrome, lupic syndrome, acute pancreatitis.1,4 Being metabolised in the liver into major and minor metabolites, both INH and its metabolites are potential candidates to explain the mechanisms responsible for the hypersensitivity reactions, not totally understood – there are some published cases of possible IgE mediated hypersensitivity and delayed hypersensitivity.2,4 Diagnostic tests for immediate-type reactions are not standardised. In this case we performed skin testing to INH based on published recommendations,4 and obtained negative results. The type and reproducibility of our patient's symptoms upon re-exposure and the eosinophilia favours our suspicion of an immunological situation. Our protocol was adapted from published cases.2–5
RIF is a semi-synthetic agent of the group of rifamycin antibiotics, metabolised in the liver. ADR are reported in up to 20% of patients,3,4 and can be either toxic (mostly gastrointestinal and hepatic) or immunological – (i) hypersensitivity reactions type I, IgE-mediated – urticaria, dyspnoea, hypotension, anaphylaxis; (ii) type II, with auto-antibodies to RIF – allergic respiratory syndrome, acute renal failure, haemolytic anaemia; (iii) type III, mediated by immune complexes – flu-like syndrome, serum sickness.1,4 However, hypersensitivity reactions are rare and are usually observed during intermittent or discontinuous regimens.1,4 As previously described regarding INH, the reproducibility of the positive responses (with anaphylactic shock) upon re-exposure, as well as the positive immediate intradermal skin test (based on published recommendations4), are consistent with an IgE-mediated hypersensitivity reaction (type I), although we cannot exclude a false positive result due to the irritant characteristics of RIF.3 Our protocol was adapted from published cases.2–5 The generalised rash which occurred 2h after the beginning of the protocol (cumulative dosage: 31.6mg) was easily controlled with hydroxizine and would not have been impeditive of proceeding with the protocol if it were not for the Infectiology Unit medical staff's decision, who decided to suspend it.
PZA is a synthetic pyrazine analogue of nicotinamide, responsible for most of the ADR among antituberculosis agents.1 These reactions are mainly toxic and may affect several organ-systems: liver (cytolysis), articulations (arthralgia), gastrointestinal system (nausea, vomiting, diarrhoea, abdominal pain, anorexia). PZA is also associated to skin reactions (early-onset maculopapular generalised pruriginous rash, sometimes accompanied by dyspnoea and abdominal pain, suggesting an anaphylactic/anaphylactoid mechanism; polymorphic erythema; phototoxicity; acne; pellagra), isolated fever or hypersensitivity drug syndrome,1 which seems to be the case of our patient. The mechanisms underlying these reactions are undetermined.4 The doubts concerning the possible immunological mechanisms of our patient's reaction to PZA vanished when he developed a generalised rash during the tolerance induction protocol. This reaction was easily controlled and, after some adjustments, we were able to conclude the protocol.
Regarding EMB, the most frequent ADR related to this drug is ocular toxicity. Other possible reactions are rare: skin allergic reactions (0.5%) such as toxic epidermal necrolysis, morbilliform eruptions and purpuric lesions; drug fever (0.3%); dyspnoea; pulmonary infiltrates; hyperbilirrubinaemia; eosinophilia; neutropenia; thrombocytopenia; acute renal failure; anaphylaxis; neuropathy.1,4 The mechanisms underlying these reactions are unknown. We cannot establish a definite causal relationship between EMB and any of the clinical events of our patient, given the fact that this drug was never introduced separately. Still, for safety reasons, and being unable to rule out its involvement on the reactions, we followed a controlled administration protocol. Our protocol was based on published recommendations1,4 and was administered with no intercurrences.
As for the slight elevation of hepatic transaminases, which began after the 10th day of therapy and was completely resolved within 2 months of therapy, it can be attributed to any of the drugs administered, although the most probable ones are INH, PZA or RIF. The mechanisms involved are probably non-immunological and if it had recurred upon re-introduction of any of the drugs (which was not the case) it would have led to its suspension.
This case illustrates the benefits of rapid oral tolerance induction in hypersensitivity drug reactions to multiple drugs, especially when dealing with a serious and potentially fatal pathology such as pulmonary tuberculosis, guaranteeing the success of the patient's therapy and his cure. This case also highlights the variability of the hypersensitivity mechanisms observed with antituberculosis agents, as well as the difficulty regarding diagnostic tests and methods for immunological confirmation, already described in similar cases published in literature.1–3