Cadmium (Cd) is a toxic heavy metal and the components of tobacco and scalp hair effectively reflect a long-term environmental exposure.
ObjectiveThe aim of this study was to assess the concentration of Cd levels in the hair of children with recurrent wheezing, and to evaluate the predictors of elevated Cd levels with a focus on anthropometric, environmental, and dietary factors.
MethodsIn this case-control study, scalp hair was obtained from 65 children with recurrent wheezing (RW) and from 65 healthy children (HC). Hair Cd concentrations were determined by ICP-MS.
ResultsMedian (IQR) hair Cd levels were 0.22μg/kg (0.10–0.35) in RW group and 0.12μg/kg (0.04–0.23) in HC group (p=0.013). Multivariable logistic regression model results showed that being a child with RW (OR=6.28; p=0.001), ETS exposure at home (OR=22.56; p<0.001), and mother's education level (OR=0.49; p=0.020), are the major predictor variables for elevated hair Cd levels (cut off >0.17μg/kg). In RW group, multivariable logistic regression results showed that hair Cd levels of >0.17μg/kg was significantly predictive of having three or more wheezing episodes in RW group after adjustment for ETS exposure at home (OR=5.48; p=0.012).
ConclusionWe demonstrated that the more children are exposed to ETS at home, the more they are exposed to heavy metals like Cd. Especially children who have had three or more wheezing attacks over the last six months are much more susceptible than the other asthmatic and non-asthmatic children, and Cd exposure aggravates their asthmatic status.
Heavy metal pollution has become a serious health concern in recent years. Cadmium (Cd) is a toxic heavy metal and the components of tobacco, together with water and food contamination, represent the main sources of non-occupational exposure in the general population.1,2 Cd enters the human body mainly after inhalation of environmental tobacco smoke (ETS) and also via gastrointestinal absorption. Inhalation is the major means of cadmium absorption for children.3,4 Continuous exposure to low levels of Cd may result in bioaccumulation and can cause a variety of adverse health effects on human beings, depending on the level and the duration of exposure, among which kidney dysfunction, lung diseases and disturbed calcium metabolism and bone defects are the most prominent.5,6
However, trace element and toxic heavy metal concentrations in biological samples are affected by environmental and geographical factors. Therefore, considerable variations can occur between specific subgroups of the populations.6 Another difficulty in assessing the effects of toxic heavy metals for past and long-term exposure is to find a reliable biological indicator.7 Cd in blood is widely used as indices for current or recent exposure.8,9 Compared to other types of clinical specimens, scalp hair has different uses and even advantages over blood or urine,8 and each centimetre of scalp hair reflects approximately one month of past exposure.9 A considerable number of studies have revealed that head hair better reflects a long-term environmental exposure than does blood and/or urine for toxic metals like Cd (as biomarker), and children's head hair may serve as a first biochemical indicator for assessment of total Cd environmental exposure.7,10–12 Although there are many studies which presently investigate hair toxic metal levels in children and adults, there is no study which has investigated whether being an asthmatic is a risk factor for elevated hair Cd levels or not. Moreover, up to now there has been no study to investigate hair Cd levels of children with recurrent wheezing.
We performed a case-control study to assess the concentrations of Cd in hair of children with recurrent wheezing and also to evaluate the predictors of elevated Cd levels with a view to anthropometric, environmental, and dietary factors. We hypothesised that the level of hair Cd in children with recurrent wheezing would probably be higher than that of healthy children.
MethodsStudy populationThis case-control study is comprised of 65 children (23 girls and 42 boys) between the ages of 1 and 6 years with a mean of 35.46±18.94 months, who were treated for recurrent wheezing (RW) in the outpatient section, Department of Pediatric Allergy, Kecioren Education and Research Hospital, Turkey, between December 1, 2007 and January 31, 2008. The exclusion criteria are as follows: (1) respiratory tract infections or wheezing episode in the last 30 days; (2) malnutrition (weight for age<90% of expected); (3) systemic corticosteroid use in the last 30 days; (4) chronic lung diseases including cystic fibrosis; (5) immunodeficiency; (6) known renal or hepatic dysfunction; (7) anatomic abnormalities of the respiratory tract; (8) suspected foreign body aspiration or croup; (9) immunosuppressive or immunostimulant treatment. Also, 65 age- and sex-matched healthy children (HC) with the mean age of 38.49±16.33 months were included in the study as a control group. None of them had ever wheezy or infectious disease for one month and none of them had atopy. The study was approved by the Ethics Committee of the Ministry of Health of Turkey, and written informed consent was obtained from the parents in each case during the enrolment process. After written informed consent was obtained, a 5–10min face-to-face interview was conducted with the mother about: (a) the mother's educational background; (b) total income of the family; (c) smoking status at home; (d) number of respiratory tract infections (RTIs) and wheezing episodes that children may have suffered in the past six months; and (e) family history of asthma and atopy. In the RW group, the number of RTIs and wheezing episodes in the past six months have been obtained from the records taken from the out-patient clinic.
The economic classification of families was designed according to the total income of parents, and their economic status was then categorised into four groups according to their income in Euros/per month (1=≤500, 2=501–1000, 3=1001–1500, 4=>1500). Educational classification was conducted according to the education level of mothers and they were classified into four main categories: primary, secondary, high school, and university graduates.
Atopy was defined according to positive skin prick test result (wheal diameter at least 3mm greater than negative control) and also according to one of the regional allergens.
A food frequency questionnaire was self-administered by the parent. We evaluated variables on dietary habits for shellfish (>once a month), sausage, vegetables, cabbage and carrot (1+/week), dairy products, cereals and fruits (1+/day). The questionnaire also included variables on the regular use of vitamins and minerals (including iron, beta-carotene, zinc, selenium, and multivitamins).
Heavy metal concentrations in hair samplesSamples collectionScalp hair samples weighing approximately 1.0g were taken from the occipital region of the head, using stainless steel scissors, the blades of which are not made of vanadium, and the samples are to be stored in plastic bags. To prepare the samples for analysis, the hairs were cut into 5-mm pieces and washed according to the procedure recommended by the International Atomic Energy Agency (IAEA) Advisory Group (by applying acetone and washing thrice under water to remove surface dirt and grease.13 The washed samples were then dried at about 50°C to constant weight.
Analytical methodsThe mineral element considered in this study was Cd. The mineral content was measured by inductively coupled plasma mass spectrometry (ICP-MS) following wet ashing of the organic matter according to the method of Granero et al.14 Each sample (app. 0.25g) was digested in 5ml of 65% nitric acid (Suprapur, Merck, Darmstadt, Germany) at room temperature in Teflon. All samples were heated at 200°C, 500psi during 30min for microwave digestion (MARS 5, CEM Corp., Matthews, NC, USA).
The solutions were filtered and made up to 25ml with deionised water. The accuracy of the instrumental methods was validated by replicating all samples as well as by taking measurements of a reference material every ten samples. Quantification was based on the most abundant isotope of each element free of analytical interference. Mean recovery rates were between 90% and 95%. The levels of Cd were measured by Agilent 7500 ICP-MS (Agilent Technologies, Santa Clara, CA, United States) using the isotope Cd114. The emission lines and isotopes used are free of spectral interference for this matrix type, and therefore, no further corrections were required.
Statistical analysisData analysis was performed using Statistical Package for Social Sciences (SPSS) version 11.5 software (SPSS Inc., Chicago, IL, United States). Whether the metric discrete variables were shown as mean±standard deviation or median (minimum−maximum), categorical variables were expressed as the number of cases and their percentages in other cases. The outcome variables of primary interest were hair Cd levels. Chi-square test was used to assess the statistical significance of differences between groups in the frequency distribution of categorical variables, so long as the expected cell size was not to be less than five, in which case Fisher's exact test was used. On the other hand, means were compared by Student's t test, otherwise, Mann-Whitney U test was applied for the comparison of the median values.
Multivariable logistic regression analyses were used to control for potential confounding effects. We used the likelihood ratio approach to determine the best-fit multivariable model. Crude and adjusted odds ratio (OR) for children with hair Cd levels were determined according to median values of (cut off) hair Cd level is 0.17μg/kg. Predictor variables from Table 2 for Cd with a level of significance of less than 25% (p<0.25) for the unadjusted odds ratios in univariable test were included as the candidate predictors in the multivariable logistic regression model. Odds ratio and 95% confidence intervals for all independent variables were also calculated. A p value less than 0.05 was considered statistically significant.
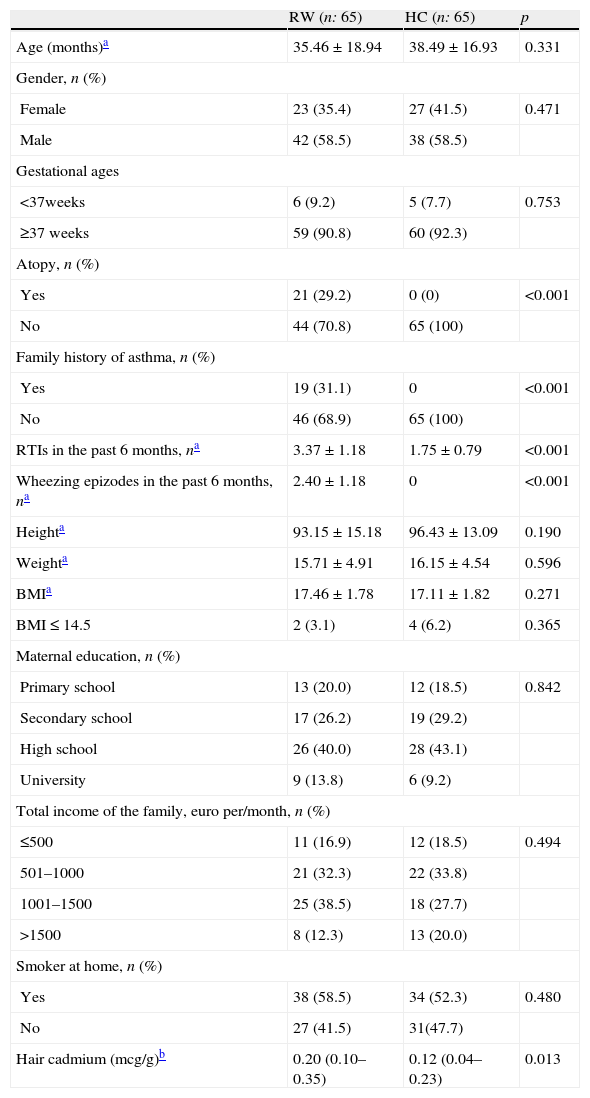
ResultsPatientsSeventy-four children as the recurrent wheezing (RW) group and 71 healthy children as the healthy control (HC) group were invited to participate in the study, and 65 children in each group were included in the study. A total of nine patients in the RW group and six children in the HC group could not participate in the study because of very short hair and/or not accepting the protocol. No statistically meaningful differences were found in baseline characteristics between the two groups for age, gender, height, weight, sociodemographic characteristics (mother's education levels and total income of the family) and ETS exposure at home. Atopy, family history of asthma, number of RTIs and wheezing episodes in the past six months were higher in the RW group. General and sociodemographic characteristics of the children in the two groups are shown in Table 1.
General and sociodemographic characteristics of the study population.
| RW (n: 65) | HC (n: 65) | p | |
| Age (months)a | 35.46±18.94 | 38.49±16.93 | 0.331 |
| Gender, n (%) | |||
| Female | 23 (35.4) | 27 (41.5) | 0.471 |
| Male | 42 (58.5) | 38 (58.5) | |
| Gestational ages | |||
| <37weeks | 6 (9.2) | 5 (7.7) | 0.753 |
| ≥37 weeks | 59 (90.8) | 60 (92.3) | |
| Atopy, n (%) | |||
| Yes | 21 (29.2) | 0 (0) | <0.001 |
| No | 44 (70.8) | 65 (100) | |
| Family history of asthma, n (%) | |||
| Yes | 19 (31.1) | 0 | <0.001 |
| No | 46 (68.9) | 65 (100) | |
| RTIs in the past 6 months, na | 3.37±1.18 | 1.75±0.79 | <0.001 |
| Wheezing epizodes in the past 6 months, na | 2.40±1.18 | 0 | <0.001 |
| Heighta | 93.15±15.18 | 96.43±13.09 | 0.190 |
| Weighta | 15.71±4.91 | 16.15±4.54 | 0.596 |
| BMIa | 17.46±1.78 | 17.11±1.82 | 0.271 |
| BMI≤14.5 | 2 (3.1) | 4 (6.2) | 0.365 |
| Maternal education, n (%) | |||
| Primary school | 13 (20.0) | 12 (18.5) | 0.842 |
| Secondary school | 17 (26.2) | 19 (29.2) | |
| High school | 26 (40.0) | 28 (43.1) | |
| University | 9 (13.8) | 6 (9.2) | |
| Total income of the family, euro per/month, n (%) | |||
| ≤500 | 11 (16.9) | 12 (18.5) | 0.494 |
| 501–1000 | 21 (32.3) | 22 (33.8) | |
| 1001–1500 | 25 (38.5) | 18 (27.7) | |
| >1500 | 8 (12.3) | 13 (20.0) | |
| Smoker at home, n (%) | |||
| Yes | 38 (58.5) | 34 (52.3) | 0.480 |
| No | 27 (41.5) | 31(47.7) | |
| Hair cadmium (mcg/g)b | 0.20 (0.10–0.35) | 0.12 (0.04–0.23) | 0.013 |
BMI: body mass index; RTIs: respiratory tract infections; RW: recurrent wheezing; HC: healthy control.
Median (25–75%) hair Cd levels were 0.22μg/kg (0.10–0.35) in the RW group and 0.12μg/kg (0.04–0.23) in the HC group (p: 0.013).
Median (25–75%) hair Cd levels of children who have positive history of ETS exposure at home are higher than children who have negative history of smoking at home in the RW group [0.32 (0.22–0.56) vs. 0.11 (0.01–0.17), respectively, p<0.001].
Median (25–75%) hair Cd levels of children who have positive history of ETS exposure at home are higher than children who have negative history of smoking at home in the HC group [0.20 (0.13–0.46) vs. 0.06 (0.01–0.13), respectively, p: 0.002].
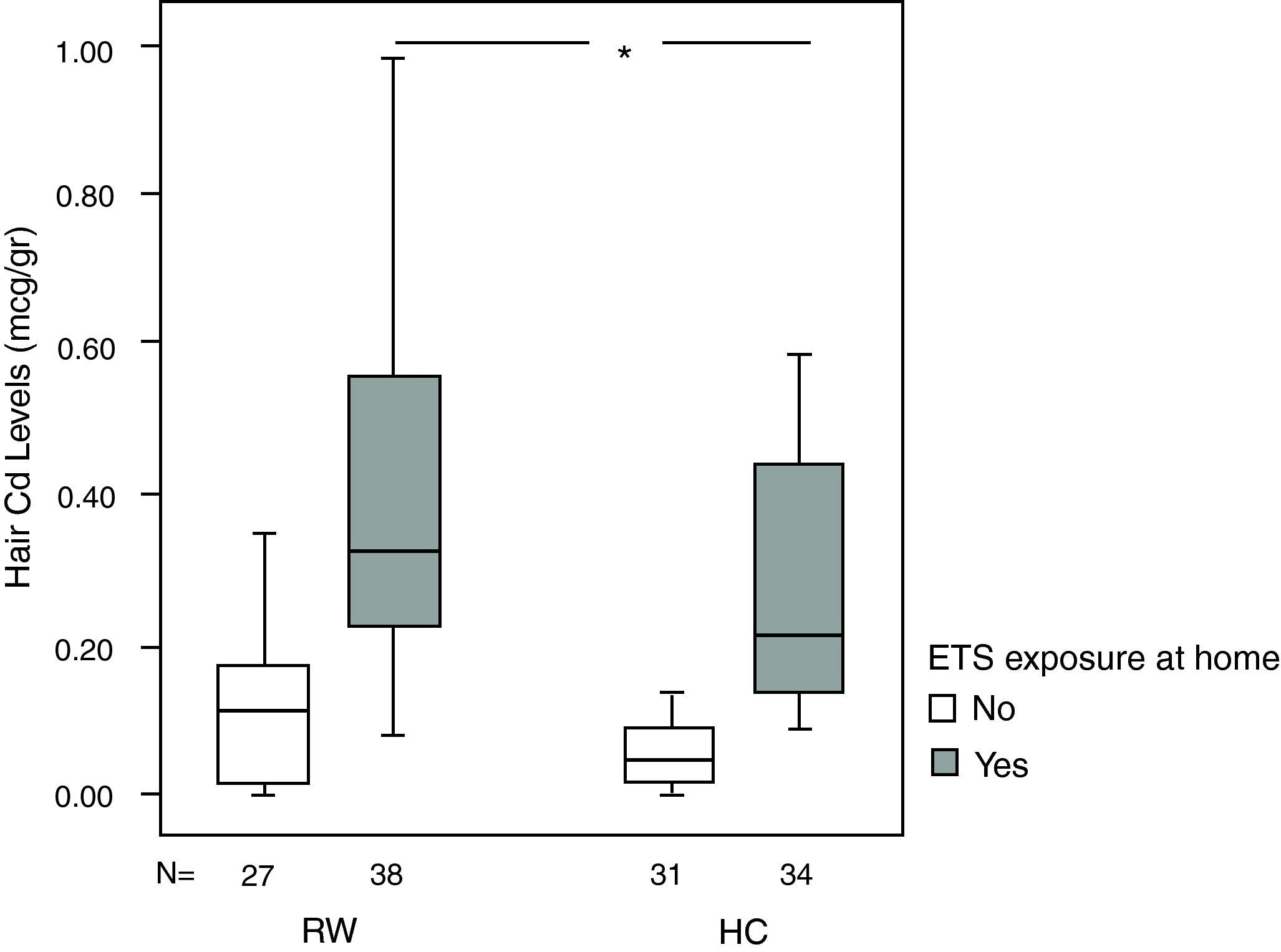
Although hair Cd levels are higher in the RW group with respect to the HC group [0.11 (0.01–0.17) vs. 0.06 (0.01–0.13), respectively, p=0.131] in children with negative history of ETS exposure at home, the difference is significant only in children with positive history of ETS exposure at home [0.32 (0.22–0.56) vs. 0.20 (0.13–0.46), respectively, p=0.009] (Fig. 1).
Median hair Cd levels according to smoking status in the two groups. Although hair Cd levels are higher in RW group with respect to HC group in children with negative history of ETS exposure at home [0.11 (0.01–0.17) vs. 0.06 (0.01–0.13), p=0.131], difference is significant only in children with positive history of ETS exposure at home [0.32 (0.22–0.56) vs. 0.20 (0.13–0.46), p=0.009]. Data points are median values, the horizontal lines of the boxes represent the 25th, 50th and 75th percentiles of the variables and the brackets represent minimum to maximum levels. p values represent between group comparisons. *p=0.009 (Mann-Whitney U test).
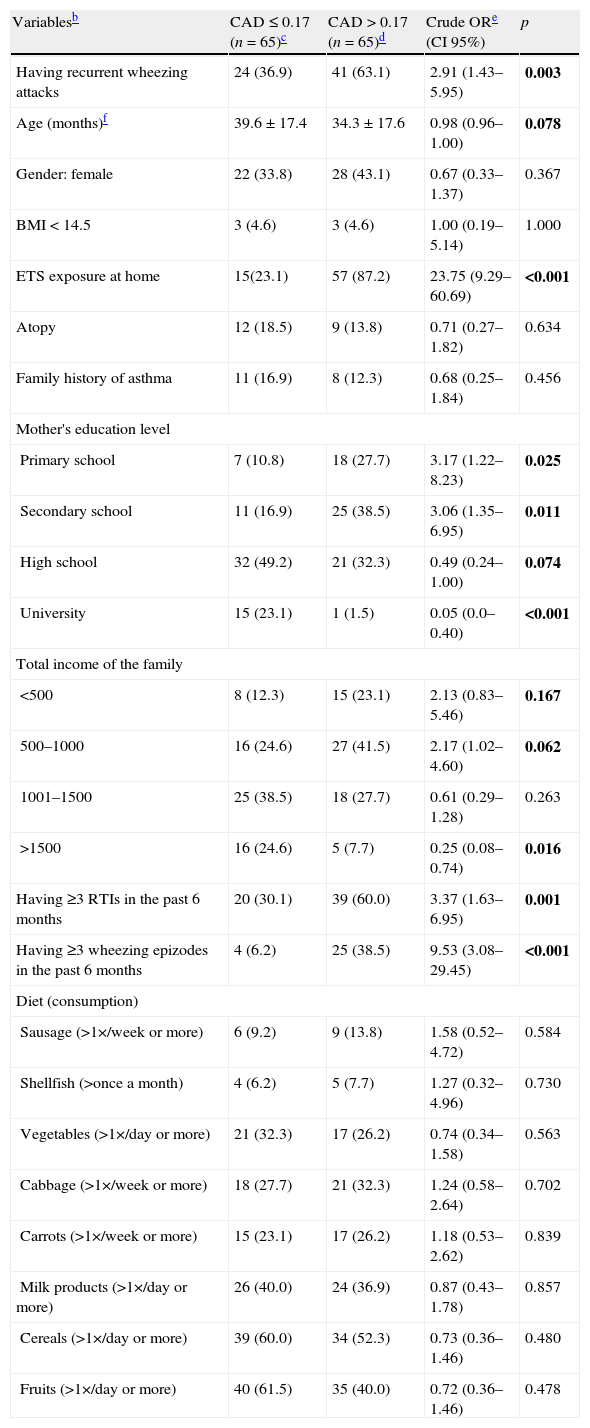
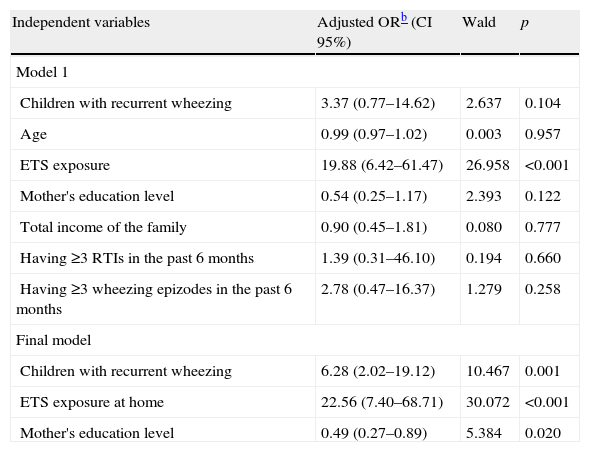
Table 2 presents unadjusted odds ratios for children with hair Cd levels >0.17μg/kg (over median level) in univariate tests. Having recurrent wheezing attacks, age, exposure to ETS at home, mother's education level, total income of the family, having three or more RTIs and having three or more wheezing episodes in the last six months were included in the study since they were regarded as candidate predictors in the multivariate logistic regression model (Table 3). Final multivariate logistic regression model results showed that being a child with RW (OR=6.28; p=0.001), ETS exposure at home (OR=22.56; p<0.001) and mother's education level (OR=0.49; p=0.020), are the major predictor variables for elevated hair Cd levels (>0.17μg/kg).
Predictors associated with elevated hair cadmium levels (>0.17μg/kg)a in univariable analyses in children with RW (n=65) and HC (n=65).
| Variablesb | CAD≤0.17 (n=65)c | CAD>0.17 (n=65)d | Crude ORe (CI 95%) | p |
| Having recurrent wheezing attacks | 24 (36.9) | 41 (63.1) | 2.91 (1.43–5.95) | 0.003 |
| Age (months)f | 39.6±17.4 | 34.3±17.6 | 0.98 (0.96–1.00) | 0.078 |
| Gender: female | 22 (33.8) | 28 (43.1) | 0.67 (0.33–1.37) | 0.367 |
| BMI<14.5 | 3 (4.6) | 3 (4.6) | 1.00 (0.19–5.14) | 1.000 |
| ETS exposure at home | 15(23.1) | 57 (87.2) | 23.75 (9.29–60.69) | <0.001 |
| Atopy | 12 (18.5) | 9 (13.8) | 0.71 (0.27–1.82) | 0.634 |
| Family history of asthma | 11 (16.9) | 8 (12.3) | 0.68 (0.25–1.84) | 0.456 |
| Mother's education level | ||||
| Primary school | 7 (10.8) | 18 (27.7) | 3.17 (1.22–8.23) | 0.025 |
| Secondary school | 11 (16.9) | 25 (38.5) | 3.06 (1.35–6.95) | 0.011 |
| High school | 32 (49.2) | 21 (32.3) | 0.49 (0.24–1.00) | 0.074 |
| University | 15 (23.1) | 1 (1.5) | 0.05 (0.0–0.40) | <0.001 |
| Total income of the family | ||||
| <500 | 8 (12.3) | 15 (23.1) | 2.13 (0.83–5.46) | 0.167 |
| 500–1000 | 16 (24.6) | 27 (41.5) | 2.17 (1.02–4.60) | 0.062 |
| 1001–1500 | 25 (38.5) | 18 (27.7) | 0.61 (0.29–1.28) | 0.263 |
| >1500 | 16 (24.6) | 5 (7.7) | 0.25 (0.08–0.74) | 0.016 |
| Having ≥3 RTIs in the past 6 months | 20 (30.1) | 39 (60.0) | 3.37 (1.63–6.95) | 0.001 |
| Having ≥3 wheezing epizodes in the past 6 months | 4 (6.2) | 25 (38.5) | 9.53 (3.08–29.45) | <0.001 |
| Diet (consumption) | ||||
| Sausage (>1×/week or more) | 6 (9.2) | 9 (13.8) | 1.58 (0.52–4.72) | 0.584 |
| Shellfish (>once a month) | 4 (6.2) | 5 (7.7) | 1.27 (0.32–4.96) | 0.730 |
| Vegetables (>1×/day or more) | 21 (32.3) | 17 (26.2) | 0.74 (0.34–1.58) | 0.563 |
| Cabbage (>1×/week or more) | 18 (27.7) | 21 (32.3) | 1.24 (0.58–2.64) | 0.702 |
| Carrots (>1×/week or more) | 15 (23.1) | 17 (26.2) | 1.18 (0.53–2.62) | 0.839 |
| Milk products (>1×/day or more) | 26 (40.0) | 24 (36.9) | 0.87 (0.43–1.78) | 0.857 |
| Cereals (>1×/day or more) | 39 (60.0) | 34 (52.3) | 0.73 (0.36–1.46) | 0.480 |
| Fruits (>1×/day or more) | 40 (61.5) | 35 (40.0) | 0.72 (0.36–1.46) | 0.478 |
Bold variables demonstrate predictor variables with a level of significance of less than 25% (p < 0.25) for the unadjusted odds ratios in univariable analyses which were included as the candidate predictors in the multivariable logistic regression model.
BMI: body mass index; ETS: environmental tobacco smoke; RTIs: respiratory tract infections.
Multivariable logistic regression models results of all predictors associated with hair Cd levels (>0.17μg/kg)a obtained from univariable analyses.
| Independent variables | Adjusted ORb (CI 95%) | Wald | p |
| Model 1 | |||
| Children with recurrent wheezing | 3.37 (0.77–14.62) | 2.637 | 0.104 |
| Age | 0.99 (0.97–1.02) | 0.003 | 0.957 |
| ETS exposure | 19.88 (6.42–61.47) | 26.958 | <0.001 |
| Mother's education level | 0.54 (0.25–1.17) | 2.393 | 0.122 |
| Total income of the family | 0.90 (0.45–1.81) | 0.080 | 0.777 |
| Having ≥3 RTIs in the past 6 months | 1.39 (0.31–46.10) | 0.194 | 0.660 |
| Having ≥3 wheezing epizodes in the past 6 months | 2.78 (0.47–16.37) | 1.279 | 0.258 |
| Final model | |||
| Children with recurrent wheezing | 6.28 (2.02–19.12) | 10.467 | 0.001 |
| ETS exposure at home | 22.56 (7.40–68.71) | 30.072 | <0.001 |
| Mother's education level | 0.49 (0.27–0.89) | 5.384 | 0.020 |
RTIs: respiratory tract infections; ETS: environmental tobacco smoke.
In addition, the following foods were not associated with elevated hair Cd levels: shellfish, sausages, vegetables, cabbage, carrots, dairy products, cereals and fruits.
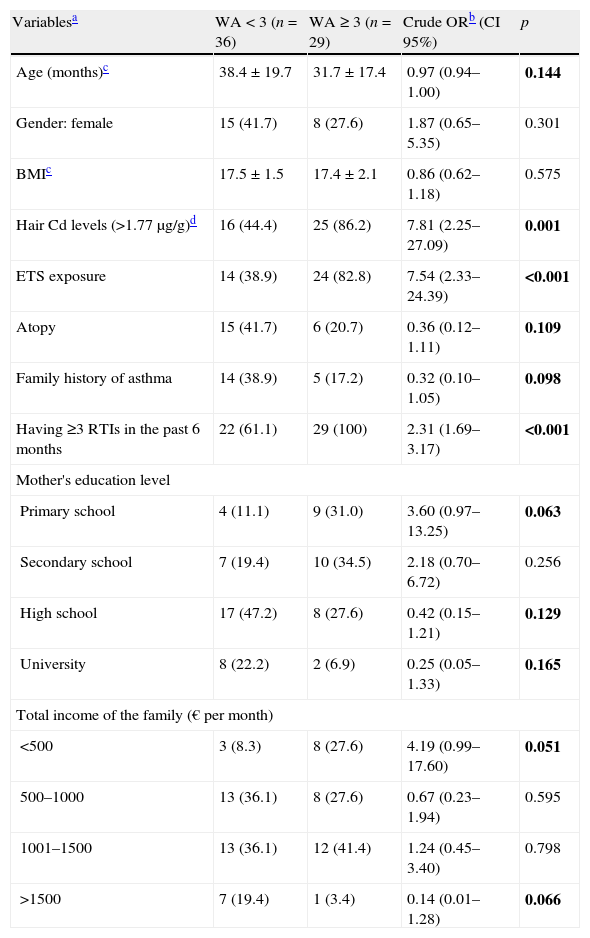
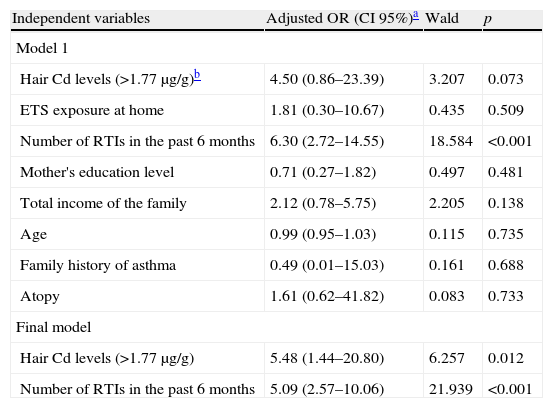
Subgroup analysesSince being a children with recurrent wheezing and ETS exposure at home were the main predictors for elevated hair Cd levels, we performed a subgroup analysis to determine whether higher hair Cd levels and/or ETS exposure at home have any effect on having three or more wheezing episodes in the past six months in the RW group. Table 4 presents unadjusted odds ratios for children with three or more wheezing episodes in the past six months in univariate tests. Hair Cd levels >0.17μg/g (over median value), ETS exposure at home, having three or more RTIs in the past six months, mother's education level, total income of the family, age, family history of asthma and atopy were included as candidate predictors in the multivariate logistic regression model. In Table 5 final multivariate logistic regression results showed that hair Cd levels of >0.17μg/kg (over median level) were significantly predictive of having three or more wheezing episodes in the past six months in the RW group after adjustment for ETS exposure at home (OR=5.48; p=0.012).
Predictors associated with three or more wheezing attacks (WA) in the past six months in univariable analyses in children with RW (n=65).
| Variablesa | WA<3 (n=36) | WA≥3 (n=29) | Crude ORb (CI 95%) | p |
| Age (months)c | 38.4±19.7 | 31.7±17.4 | 0.97 (0.94–1.00) | 0.144 |
| Gender: female | 15 (41.7) | 8 (27.6) | 1.87 (0.65–5.35) | 0.301 |
| BMIc | 17.5±1.5 | 17.4±2.1 | 0.86 (0.62–1.18) | 0.575 |
| Hair Cd levels (>1.77μg/g)d | 16 (44.4) | 25 (86.2) | 7.81 (2.25–27.09) | 0.001 |
| ETS exposure | 14 (38.9) | 24 (82.8) | 7.54 (2.33–24.39) | <0.001 |
| Atopy | 15 (41.7) | 6 (20.7) | 0.36 (0.12–1.11) | 0.109 |
| Family history of asthma | 14 (38.9) | 5 (17.2) | 0.32 (0.10–1.05) | 0.098 |
| Having ≥3 RTIs in the past 6 months | 22 (61.1) | 29 (100) | 2.31 (1.69–3.17) | <0.001 |
| Mother's education level | ||||
| Primary school | 4 (11.1) | 9 (31.0) | 3.60 (0.97–13.25) | 0.063 |
| Secondary school | 7 (19.4) | 10 (34.5) | 2.18 (0.70–6.72) | 0.256 |
| High school | 17 (47.2) | 8 (27.6) | 0.42 (0.15–1.21) | 0.129 |
| University | 8 (22.2) | 2 (6.9) | 0.25 (0.05–1.33) | 0.165 |
| Total income of the family (€ per month) | ||||
| <500 | 3 (8.3) | 8 (27.6) | 4.19 (0.99–17.60) | 0.051 |
| 500–1000 | 13 (36.1) | 8 (27.6) | 0.67 (0.23–1.94) | 0.595 |
| 1001–1500 | 13 (36.1) | 12 (41.4) | 1.24 (0.45–3.40) | 0.798 |
| >1500 | 7 (19.4) | 1 (3.4) | 0.14 (0.01–1.28) | 0.066 |
Bold variables demonstrate predictor variables with a level of significance of less than 25% (p < 0.25) for the unadjusted odds ratios in univariable analyses which were included as the candidate predictors in the multivariable logistic regression model.
BMI: body mass index; RTIs: respiratory tract infections.
Multivariable logistic regression models results of all predictors associated with three or more wheezing attacks in the past six months obtained from univariable analyses in children with RW (n=65).
| Independent variables | Adjusted OR (CI 95%)a | Wald | p |
| Model 1 | |||
| Hair Cd levels (>1.77μg/g)b | 4.50 (0.86–23.39) | 3.207 | 0.073 |
| ETS exposure at home | 1.81 (0.30–10.67) | 0.435 | 0.509 |
| Number of RTIs in the past 6 months | 6.30 (2.72–14.55) | 18.584 | <0.001 |
| Mother's education level | 0.71 (0.27–1.82) | 0.497 | 0.481 |
| Total income of the family | 2.12 (0.78–5.75) | 2.205 | 0.138 |
| Age | 0.99 (0.95–1.03) | 0.115 | 0.735 |
| Family history of asthma | 0.49 (0.01–15.03) | 0.161 | 0.688 |
| Atopy | 1.61 (0.62–41.82) | 0.083 | 0.733 |
| Final model | |||
| Hair Cd levels (>1.77μg/g) | 5.48 (1.44–20.80) | 6.257 | 0.012 |
| Number of RTIs in the past 6 months | 5.09 (2.57–10.06) | 21.939 | <0.001 |
ETS: environmental tobacco smoke; RTIs: respiratory tract infections.
Odds ratios for the outcome variable (three or more wheezing attacks in the past six months) for each predictor variable, adjusted for all other variables. CI indicates confidence interval. Global final model with above two variables has a likelihood ratio of G2=62.61 (d.f. 2), p<0.001. All variables in logistic regression model are dichotomous.
In this study we demonstrated that hair Cd levels were higher in children with recurrent wheezing than those in the healthy controls. Our results showed that exposure to ETS at home was the major predictor for elevated hair Cd levels both in asthmatic and non-asthmatic children. Moreover, this is the first study in the literature showing a strong association between elevated hair Cd levels and being asthmatic children.
The most common sources of environmental cadmium exposure are diet and smoking. Of total ingested cadmium, approximately 5% is absorbed in adults. However, the mechanism of absorption is not well understood in children.15 Some studies have indicated that dietary sources of cadmium include organ meats,16 shellfish,17 and potatoes and leafy vegetables.18 But in many studies the relationship with hair, blood and urinary Cd levels and diet could not be demonstrated.1,15,19 However, Friedman15 demonstrated that there seem to be higher blood Cd levels among children reportedly eating sausage once a week or more. In our study no relationship has been indicated between hair Cd levels and diet.
Because cadmium is more efficiently absorbed through the lungs than it is through the gastrointestinal tract,16 smoking poses a serious risk. Each cigarette contains varying amounts of Cd. A cigarette generally contains 1–2μg Cd.20,21 About 10–50% of it goes into the lungs and is absorbed depending on the particle size and the remainder is emitted into the atmosphere to be inhaled by others or to contaminate the environment.22 In many studies it was demonstrated that (active) smokers have much higher blood Cd concentrations.23,24 In another study it was demonstrated that children with asthma who are exposed to ETS have elevated urinary cotinine and urinary Cd levels and urinary Cd correlated well with urinary cotinine which is used as a marker of tobacco smoke exposure in smokers.25 Özden et al.12 demonstrated that household exposure to smoking and attending a school situated near highways were found to be the most important risk factors for the high hair cadmium and lead levels in healthy children. Willers et al.25 demonstrated that a major source for Cd exposure is cigarette smoking and ETS exposure may be the most important determinant of Cd status in children. In agreement with the literature, we found in our study that exposure to ETS at home was the most important risk factors for elevated hair Cd levels. Parallel to our finding, Cd levels have been reported to be at significantly higher levels in sidestream smoke26 than in mainstream smoke.27,28 Also Willers et al.25 showed 20–50% of ETS may have been absorbed in the lung due to finely dispersed particles. These evidences demonstrated that Cd accumulates not only in smokers29,30 but also in non-smokers who are exposed to ETS, and children are particularly at risk due to their higher respiratory rate which results in increased inhalation of ETS and related toxic metals.
Hemdan et al. showed that short-term exposure of monoclonal antibody-stimulated cells to low Cd doses may cause inhibition of secretion of interleukin (IL)-1β greatly, tumour necrosis factor (TNF)-α and interferon (IFN)-γ was compared to production of IL-4 and IL-10.31 This type-2-based immune response indicates a shift of the immune response from type-1 to -2.31 They speculated, in accordance with the literature, that this may increase the incidence of allergic diseases.32–34 Although in the RW group hair Cd levels of atopic children were slightly lower than the non-atopic ones (data not shown), we could not find any significant relationship between hair Cd levels and atopy. In accordance with this, it was shown in a study that parents with atopic children tried to preserve their kids from passive smoking35 and this may explain our findings.
Although Hossny et al. showed no relationship with asthma and elevated blood Cd levels in children,1 one of the important findings of our study was that being an asthmatic child is one of the significant predictors for elevated hair Cd levels. However, we found that elevated hair Cd levels with respect to the HC group is significant only in children who have positive history of ETS exposure at home. It is well known that children with ETS exposure would result in elevated Cd, and then generate wheezing. Moreover, the occurrence of ETS exposure can be observed usually before outcome (recurrent wheezing). Yet, in our study, the subjects have been included in the study after they have developed recurrent wheezing attacks, and their hair Cd levels have then been measured. As it is known, the measurement of toxic elements signifies prolonged exposures. Suffering from asthma (i.e. having RW) may be induced by two reasons in terms of their being determinant for hair Cd levels (see Table 3). This is caused by the fact that either these children are exposed to much more cigarette smoke compared to other children or that they absorb too much Cd. In our study, the rate of exposure to cigarette smoke of the children with RW and of those with HC is almost similar (see Table 1). Thus, having children with RW and its likely contribution to the increase in the levels of hair Cd might be explained through the fact that these children absorb more cigarette smoke. As a matter of fact, when only the children with RW have been included and sub-analysed in the study (Table 5), it has been observed in the final model that, of the major predictable factors leading to three or more wheezing attacks, it was the elevated hair Cd level but not ETS exposure. It is indisputable that there is a correlation between exposure to cigarette smoke and levels of hair Cd. However, when both exposure to cigarette smoke and hair Cd levels are included in the model (see Table 5), the level of elevated hair Cd, but not cigarette smoke, has been found as the determinant of three or more wheezing attacks in the final model. This supports our aforementioned view as well. Otherwise, ETS exposure should have been observed as a predictor in the final model. All of these findings led us think that there is a link between chronic Cd exposure and airway physiology. Moreover, asthmatic children absorb greater amounts of Cd than healthy children do when they are exposed to ETS, and Cd exposure via exposure to ETS aggravates asthma. There may be some explanations for these findings; maybe this is due to the higher respiratory rates of these children and/or an increased pulmonary uptake, due to small airways disease and airway inflammation in children with asthma.25 The toxicity of cadmium in the lungs has been well documented in animal studies. For example, cadmium inhalation produces a pulmonary inflammatory response in mammals.36 In accordance with this finding, Morrison et al.37 showed increased epithelial permeability which is known to occur in response to tobacco smoke exposure, leading to inflammatory responses. Balharry et al.38 investigated an in vitro study to assess toxicity of inhaled tobacco smoke components which include nicotine, cadmium, formaldehyde and urethane. They demonstrated that all of these four tobacco smoke components induced production of pro-inflammatory cytokines like IL-1α, IL-1β, IL-6 and GM-CSF.38 It is more likely that Cd exposure may alter the integrity of respiratory epithelia or mast cells, increase inflammation, and increase the severity of asthma.1 Kirschvink et al. showed that exposure to Cd for three weeks led to higher bronchoalveolar lavage fluid concentrations of macrophages and neutrophils in rats, as well as to decreased levels of glutathione during the first week of exposure.36 Also the authors suggested that cadmium exposure could lead to an acute inflammatory reaction accompanied by a build-up of free radicals (evidenced by decreased glutathione levels), which could consequently lead to lung inflammation.36 Moreover, Lampe et al. demonstrated that chronic cadmium exposure is associated with reduced pulmonary function, and cigarette smoking modifies this association.39 Animal studies also support a link between Cd exposure and reduced pulmonary function. For example, rats exposed to Cd aerosol at a concentration of 1.6mg/m3 for two weeks had increased leukocyte concentrations and alveolar thickening in the lung.40 The administration of three intratracheal injections of cadmium chloride over a five-day period was associated with decreased lung capacity and increased alveolar wall thickness in rats.41 In agreement with the evidence and in the light of our results, we speculated that Cd induced inflammation in respiratory epithelium may aggravate asthma and may cause more Cd absorption.
However, because hair Cd was associated with severity of asthmatic status (as significant predictor for three or more wheezing episodes in the RW group) even after adjusting for smoking status, smoking factors alone cannot explain the association. The Cd in cigarette smoke may exert a toxic effect that is independent of the combined effects of the non-Cd elements of cigarette smoke. Unfortunately, our study does not offer an answer to the question of whether other metals and non-metal components that are found in cigarette smoke, such as lead, aluminium and nicotine may enhance the effect of cadmium on asthmatic status or not.
In conclusion, we demonstrated that the more children are exposed to ETS at home, the more they are exposed to heavy metals like Cd. Those children who have had three or more wheezing attacks over the last six months are much more susceptible than the other asthmatic and non-asthmatic children, and Cd exposure aggravates their asthmatic status. Children should not have household exposure to smoking, especially those suffering from asthma.
Conflict of interestThe authors have no conflict of interest to declare.




![Median hair Cd levels according to smoking status in the two groups. Although hair Cd levels are higher in RW group with respect to HC group in children with negative history of ETS exposure at home [0.11 (0.01–0.17) vs. 0.06 (0.01–0.13), p=0.131], difference is significant only in children with positive history of ETS exposure at home [0.32 (0.22–0.56) vs. 0.20 (0.13–0.46), p=0.009]. Data points are median values, the horizontal lines of the boxes represent the 25th, 50th and 75th percentiles of the variables and the brackets represent minimum to maximum levels. p values represent between group comparisons. *p=0.009 (Mann-Whitney U test). Median hair Cd levels according to smoking status in the two groups. Although hair Cd levels are higher in RW group with respect to HC group in children with negative history of ETS exposure at home [0.11 (0.01–0.17) vs. 0.06 (0.01–0.13), p=0.131], difference is significant only in children with positive history of ETS exposure at home [0.32 (0.22–0.56) vs. 0.20 (0.13–0.46), p=0.009]. Data points are median values, the horizontal lines of the boxes represent the 25th, 50th and 75th percentiles of the variables and the brackets represent minimum to maximum levels. p values represent between group comparisons. *p=0.009 (Mann-Whitney U test).](https://static.elsevier.es/multimedia/03010546/0000004000000001/v1_201304101101/S0301054611000863/v1_201304101101/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)
