INTRODUCTION
Allergic reactions to food and dietary components are endemic in the industrialized world, and the number of allergens grows with the availability of new and increasingly sophisticated foodstuffs 1. At present, 6 % of the pediatric population under three years of age, and 2 % of the general population present food hypersensitivity. Eighty percent of affected children are allergic to legumes, milk and eggs 2. At the same time, frequent cross-reactions among food allergens contribute to complicate the clinical diagnosis 3.
IgE-mediated immune responses are the most widely accepted mechanism underlying allergies. Atopic individuals produce antibodies of this isotype against specific allergen epitopes, which bind to high-affinity receptors (FceRI) located in mast cells and basophils. At subsequent second exposure, the allergen interacts with these antibodies, triggering cell degranulation and the release of chemical mediators (histamine, prostaglandins, leukotrienes, etc.).
In order for this phenomenon to occur, prior sensitization implying a first exposure to the allergen is essential. Sensitization may take place in different ways, including the inhalatory, digestive and subcutaneous routes. At intestinal level, sensitization to food antigens involves the gut associated lymphoid tissue (GALT) one of the principal components of the human immune system 4,5. The intestinal mucosa contains different cellular populations that participate in immune responses: macrophages, eosinophils, mast cells, neutrophils and enteroendocrine cells, which in addition to their specific individual functions collaborate and modulate immune responses 6,7.
Macrophages form part of a large system known as the Mononuclear Phagocytic System. The components of this system are specialized migratory cells that originate in the bone marrow and circulate in blood as monocytes. The latter in turn mature and are activated within different tissues, where they are known as macrophages. These cells measure 12 to 15 m in diameter, and their granular cytoplasm contains lysosomes and phagocytic vacuoles. Unlike neutrophils, these macrophages are esterase-positive, often peroxidase negative, and contain scant glycogen 8,9.
Macrophages are normally present within the gastrointestinal tract in areas close to specialized lymphoid tissue, separated from the epithelium by a basal membrane and intimately associated to a rich network of capillaries, nerve endings and lymphatic vessels. The cells express membrane receptors for intestinal mediators and peptides (e.g., VIP, bradykinin, substance P) and release products such as prostaglandins, enzymes and cytokines (TNF, MIF, IL-1, etc.) that in turn influence the activities of neighboring cells 10-13.
Most macrophages in the intestinal mucosa exhibit a mature phenotype, though some regional subpopulations probably have different functional roles. Moreover, the cells undergo phenotypic changes in the course of inflammatory processes that can be induced by chemical mediators or may represent a recently recruited cell population 14,15.
While foreign body phagocytosis is the fundamental function of macrophages, these cells also stimulate lymphocytes, acting as antigen-presenting cells (APCs). Macrophages also constitute a critical element in the effector phase of immune reactions, and play a fundamental role in natural immunity 16,17. However, their function in IgE-dependent allergic reactions has not been fully defined to date.
In the rabbit, the cecum functions as a fermentation reservoir in a way similar to the bovine rumen, for example. The appendix is a continuation of the cecum, and constitutes the largest lymphoid organ representing half of the immune system associated to the intestinal mucosa. At intraluminal level, both organs contain bacteria and nutrients in different stages of digestion that can exert potential antigenic actions. Thus, the appendix is a very interesting model for the investigation of local immune responses 18.
In previous works we demonstrated that rabbits sensitized and orally challenged to ovalbumin (OVA) present histopathological modifications in cecal appendix mucosa, and an increase in the presence of activated T lymphocytes 19,20. The present study analyzes macrophage distribution and quantitative modifications in this same experimental model.
MATERIAL AND METHODS
Animals and sensitization
Adult male New Zealand rabbits housed under normal bioterial conditions were used. The animals were fed a balanced commercial diet (maximum 17 % raw protein and 12 % raw fiber). Both food and water were supplied ad libitum. The study protocol was approved by the local ethics committee.
The rabbits were divided into two groups of 10 animals each: G1 (non-sensitized normal controls) and G2 (rabbits sensitized by subcutaneous route with 2 ml of a solution containing 70 μg of OVA and 30 mg of aluminum hydroxide as adjuvant inoculation being carried out on two occasions spaced 15 days apart).
Serum anti-OVA IgE titers were assayed based on the PCA (passive cutaneous anaphylaxis) method, using 8-week-old Wistar rats 9. Injection of 0.05 ml of sensitized rabbit serum in increasing dilutions from 1/40 was carried out via the subcutaneous route in four different locations of the skin of the flank. The injection in the same way of non-sensitized rabbit serum was used as control. After 48 hours we administered a solution of 1 mg of OVA in 0.5 ml of phosphate buffered saline (PBS) with 1 % Evans Blue by intravenous injection. The rats were sacrificed 10 minutes after the intravenous injection. Positivity was considered in the case of dilutions that produced bluish maculae measuring 5 mm or more in diameter.
Samples and tissue fixation
The animals were anesthetized and sacrificed 15 days after the last sensitization. Samples of the appendix were obtained some being frozen in liquid nitrogen, and others being fixed in 10 % formalin buffer and Carnoy's solution.
A cryostat was used to prepare 5 μm sections of the frozen samples, followed by macrophage staining according to the alpha-naphthyl acetate esterase technique 22.
The formalin fixed specimens were embedded in paraffin and subjected to staining with hematoxylin-eosin and Masson trichromic strain for histological study. The samples immersed in Carnoy's solution were subjected to the Alcian blue technique (pH < 1) for mast cells detection.
Analysis of the results
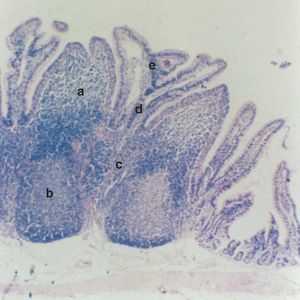
The results were quantified on the basis of 40 high-power fields (HPFs) per animal as the arithmetic mean of the esterase-positive cells per microscopic field, with the corresponding standard deviation (SD). The statistical analysis comprised the Student t-test, considering 5 regions in both study groups: A) subdomal region (subepithelial dome region); B) follicular region; C) interfollicular region; D) basal chorion (basal VIM), and E) apical chorion (apical VIM) (fig. 1).
Figure 1.--Cecal appendix of the rabbit. Note the 5 study regions: A) subdomal region (subephithelial dome region); B) follicular region, C) interfollicular region, D) basal chorion (basal VIM) and E) apical chorion (apical VIM) (x 100).
RESULTS
The PCA test proved positive at a dilution of 1/160 in group 2, and negative in all group 1 (non-sensitized) sera.
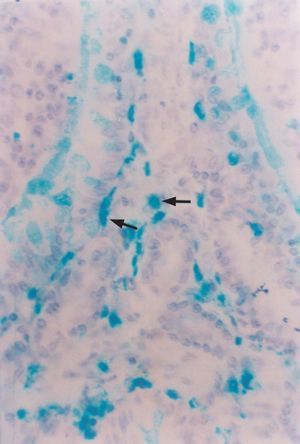
In both groups the histological study revealed lymphoid structures called appendicular patches, protruding towards the lumen. These plaques were located at mucosal and submucosal level, and were separated by crypts and tubular glands with the presence of enterocytes, enteroendocrine cells and goblet cells. The plaques were in turn lined by a simple cylindrical epithelium with basal nuclei and a pale cytoplasm, and including M cells, macrophages and lymphocytes, among other cell types. Beneath the epithelium, at plaque level, the macrophages were seen in different regions: follicular, interfollicular, subdomal, and in the lamina propria (VIM) reflecting their presence in areas that induce and execute local immune responses. In addition, an increased presence of mast cells was noted in the chorion of the villi in sensitized animals (fig. 2).
Figure 2.--Alcian blue-positive mast cells in the villous chorion of the cecal appendix. Rabbit belonging to group 2 (G2, sensitized) (x 450).
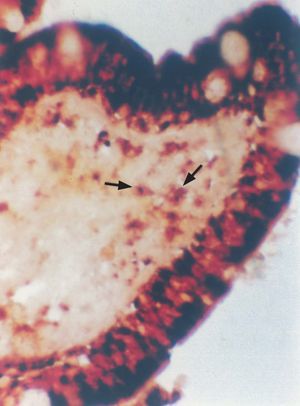
Alpha-naphthyl acetate serves as substrate for a type of nonspecific esterase abundantly found in monocyte / macrophage lysosomes, and can be used to identify and quantify these cells (table I). The epithelial M cells are also rich in alpha-naphthyl esterase; as a result, the regions corresponding to the latter were not included in the analysis (fig. 3).
Figure 3.--Esterase-positive macrophages in the subdomal region of the cecal appendix. Note the positive M cells recorded with this technique (x 450).
DISCUSSION
Although allergic disorders have been known for a long time, there is a growing interest in further clarifying the etiopathogenic factors involved in such phenomena, with the purpose of improving treatment and particularly of preventing the appearance of allergies in both children and adults.
While the importance of the monocyte/macrophage system in the pathogenesis of allergic disease remains the subject of investigation, it has been established that these mononuclear cells can become activated in the course of allergic phenomena. The intestinal macrophage population can undergo activation and may play an important role under conditions of intestinal inflammation. Many of the adverse consequences of the latter have been associated to the production of IL-1, TNF-α and IL-6 by these phagocytic cells 24.
Macrophages are able to suppress mixed lymphocyte proliferation phenomena in vitro, and under normal conditions they function as antigen-presenting cells (APCs) responsible for host tolerance responses 25. The results of the PCA (passive cutaneous anaphylaxis) technique showed the group 2 (i.e., sensitized) rabbits to produce high specific anti-OVA IgE titers indicative of important sensitization.
In earlier studies we observed an increased presence of mast cells in different segments of the digestive tube in response to sensitization thus indicating a positive relation between the immune status of the animal host and the amount of these cells 19,21.
In the present study, sensitization induced a statistically significant increase in the amount of macrophages. These results coincide with those reported by Mahida et al. in human Peyer's patches using monoclonal antibodies 25. Other studies involving biopsies from patients with intestinal inflammatory disease have recorded an increase in the mucosal population of macrophages derived from circulating monocytes 26-27.
In the appendix, the macrophage population was seen to increase in all the segments studied. As a response to sensitization, increased recruitment of circulating monocytes would take place towards the induction and effector regions of the cecal appendix. This process in turn would be induced by chemical mediators and would serve to demonstrate macrophage participation in both the effector and induction phases of local immune response.
These observations would indicate a functional relationship between the appendicular patch macrophages and the immunological conditions of the sensitized rabbit host.
ACKNOWLEDGEMENTS
The authors thank Marcela Descalzo for her technical contribution to the present study.