Due to a more wide-spread use of latex products, in particular medical gloves, allergy to natural rubber latex (NRL) had posed serious concerns during the 1980s and 90s. Currently, it is known that this epidemic of allergic reactions is especially prevalent in certain occupational groups repeatedly exposed to latex products, such as health care workers (HCW) and patients with spina bifida (SBP).1
The identification of the patients who become sensitised and are likely to suffer from symptoms upon repeated exposure to latex products is a major goal for prevention of the allergic reactions. It is generally accepted that the diagnosis must be based on the clinical history and on a confirmative assay which may include in vivo tests such as skin prick tests (SPT), provocation tests and/or laboratory-based in vitro analyses.2 Among these, SPT are described as the most reliable in vivo method for diagnosis of sensitisation to latex proteins.3 However, a potential low diagnostic sensitivity of latex SPT is associated to poorly represented and/or denatured allergens, such as Hev b 3 and Hev b 5.4,5 In fact, to perform an appropriate diagnosis, it is critical that the applied extract contains an adequate amount of all clinically relevant allergen components.4
In the management of latex allergy, specific immunotherapy (SIT) represents the most promising alternative for latex allergy treatment. Nevertheless, SIT is currently associated with a high risk of adverse events and is not available in routine clinical practice. Refined allergen preparations and administration regimens must be developed to allow widespread use. In immunotherapy extracts, unlike diagnostic extracts, the inclusion of allergens that are not clinically relevant can dilute the essential allergen components of extract and decrease its effectiveness.
Nowadays, immunotherapy and diagnosis of latex allergy are performed using NRL extracts that are obtained directly from the tree Hevea brasiliensis sap without any purification steps. This crude extract contains several hundred proteins from which 14 (Hev b 1 to Hev b 14) have been recognized by the International Union of Immunological Societies (IUIS) as latex allergens (www.allergen.org). Among the latex allergens, Hev b 1, Hev b 3, Hev b 5 and Hev b 6 are regarded as the most crucial allergens in natural rubber products.6 Here we describe a new approach to obtain and separate the four most relevant latex allergens from the major sensitisation source, the latex gloves.
Since the proteins of gloves and other NRL products are responsible for latex sensitisation, we consider that they are the ideal allergen sources of reagents for clinical purposes. Indeed, Yeang et al. reported that the latex allergens for research and clinical applications should theoretically be proteins isolated and purified from latex gloves. These researchers also noted that there are considerable problems in sourcing latex allergens from gloves because they vary widely in their protein content, both qualitatively and quantitatively. However, these problems also exist in crude extracts because there is an inherent variation in allergen content due to genetic environmental and agronomic factors.7
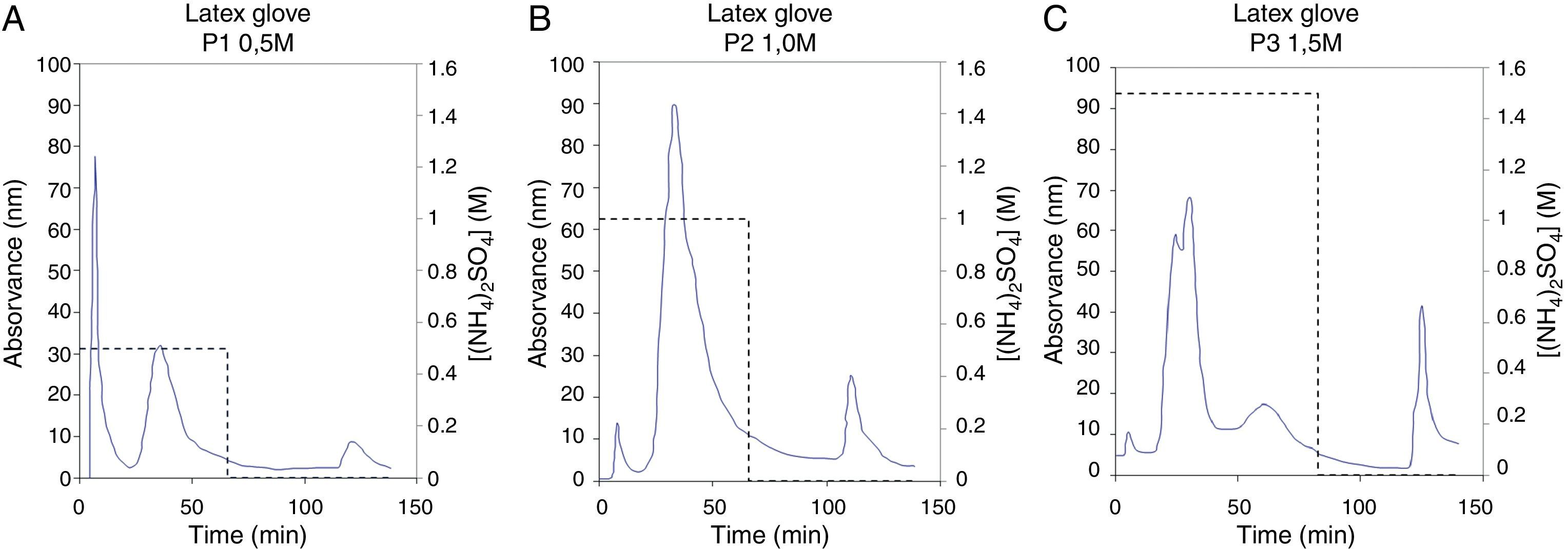
As a first step of the present method, six different glove brands were collected from health institutions in Portugal, aiming to reduce the effect of inherent protein variation that results from glove manufacture and processing. Latex proteins were extracted from the gloves and lyophilized as described previously.8 Then, 300mg of lyophilized latex glove extract were dissolved in 20mM tris–HCl, pH 7.5 and a salt precipitation was applied for both enrichment and concentration of latex allergens. This step was first performed by using 25% ammonium sulphate. After being stirred for 30min, at 4°C, the precipitated extract (denoted P1) was centrifuged at 12,000×g for 15min, at 4°C. The same process was repeated adding 50% and 75% of ammonium sulphate (extracts denoted P2 and P3, respectively). The three precipitated latex extracts (P1, P2 and P3) were then fractionated by Hydrophobic Interaction Chromatography (HIC). This technique was employed to exploit the differences in protein hydrophobicity between the four major latex allergens and to accomplish their separation. In fact, it is known that Hev b 5 and Hev b 6 have mainly hydrophilic characteristics while Hev b 1 and Hev b 3 are hydrophobic allergens.9,10 HIC was applied using a FPLC system (GE Healthcare Biosciences, Uppsala, Sweden) on a Sepharose CL-6B column modified with butyl-1,4-bis-(2,3-epoxy-propoxyd). The gel was packed in a XK column 16/20 (GE Healthcare Biosciences, Uppsala, Sweden) and equilibrated in a series of experiments using as eluent PBS 0.01M, pH 7.4 with different ammonium sulphate concentrations. The best separation conditions were achieved using 0.5M ammonium sulphate for precipitate P1, 1.0M for P2 and 1.5M for P3 (Fig. 1). At these conditions, two resolved peaks corresponding to unbound proteins were eluted (peaks 1 and 2). Bound proteins were then eluted (peak 3) by subsequently decreasing of ammonium sulphate concentration in eluent buffer to 0M, in a stepwise mode. Results showed different chromatographic profiles which suggest qualitative and quantitative differences in protein content of precipitated extracts (Fig. 1A–C).
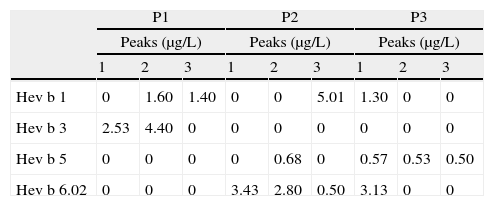
Quantification of Hev b 1, Hev b 3, Hev b 5 and Hev b 6.02 in each HIC fraction was performed by capture enzyme immunoassay (EIA) using a commercial kit (FITkit™,Icosagen, Estonia) according to the manufacturer's instructions. FITkit™ tests are based on the use of specific monoclonal antibodies developed against the four major NRL allergens, providing individual results for each NRL allergen. All assays were performed in triplicate and when allergen levels were below the detection limit they were denoted as zero (Table 1). In this quantitative analysis was observed that HIC fractionation of P1 (Fig. 1A) promoted a selective separation of hydrophobic allergens, Hev b 3 (2.53μg/L) and Hev b 1 (1.40μg/L), in peaks 1 and 3 respectively (Table 1). Moreover, HIC of P2 (Fig. 1B) showed a separation of Hev b 6.02 (3.43μg/L) in peak 1 (Table 1), whereas in peak 2 a mixture of Hev b 5 and Hev b 6.02 was obtained. It was also interesting to observe a high concentration of Hev b 1 in peak 3, despite the slight contamination of Hev b 6.02. On the other hand, separation of Hev b 5 (0.53 and 0.50μg/L) was achieved in peaks 2 and 3 (Table 1) of the HIC assay applied to the P3 precipitate (Fig. 1C). It is known that although latex sensitisation is mainly acquired at health institutions for both SBP and HCW, in general, these risk groups present different sensitisation profiles, being that Hev b 1 and Hev b 3 are considered major allergens for SBP, while Hev b 5 and Hev b 6.02 are the major allergens for HCW.1 Although the sensitisation profile of these risk groups may be more complex than this general rule, since it is common that SBP are also sensitised to Hev b 5 and Hev b 6, it is interesting to note that two different fractions containing specific allergens for each risk group were also obtained with this methodology: Peak 2 of P1 with Hev b 1 (1.60μg/L) and Hev b 3 (4.40μg/L), and Peak 2 of P2 containing Hev b 5 (0.68μg/L), Hev b 6.02 (2.80μg/L) (Table 1). These extracts and the separated allergens can be used to adjust the content of each major allergen that is present in the diagnosis reagents and also can provide a specific extracts for a more effective immunotherapy. In fact, the success of allergen-specific diagnosis and treatment is dependent on the allergen composition of the used material. It is recognized that the commercially available latex extracts vary significantly in their allergen composition and concentration and this heterogeneity could strongly affect not only the diagnosis but also the immunotherapy, due to the lack of correct identification of patient sensitisation profile and to the poor quality of the therapeutic preparations. In order to achieve balanced allergen content, commercially available diagnosis reagents should be enriched in trace latex allergens. Thus, the improvement of NRL reagents using allergen preparation such our extracts could favour the correct diagnosis of clinical allergy that is crucial to guide subsequent investigations and treatment. On the other hand, in terms of immunotherapy purposes, the complex NRL extracts should be replaced by refined protein preparations, containing the clinically relevant allergens for the sensitised patient to treat, with minimal protein contamination. In this regard, we showed that the different hydrophobicity presented by the most clinically relevant latex allergens can be exploited to obtain allergen preparations which can be useful to formulate specific immunotherapy reagents, based on the individual reactivity profile of each latex-allergic patient to treat. It is also important to stress that the minor NRL allergen content of our fractions is unknown and a high concentration of some minor allergens in each fraction cannot be excluded. For this reason, the use of such extracts in immunotherapy trials should raise safety concerns, especially in patients presenting co-sensitisation to minor NRL allergens.
Quantification of Hev b 1, Hev b 3, Hev b 5, Hev b 6.02 by EIA in latex glove fractions obtained by HIC of P1, P2 and P3 (precipitation with 25%, 50% and 75% ammonium sulphate, respectively).
| P1 | P2 | P3 | |||||||
| Peaks (μg/L) | Peaks (μg/L) | Peaks (μg/L) | |||||||
| 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | |
| Hev b 1 | 0 | 1.60 | 1.40 | 0 | 0 | 5.01 | 1.30 | 0 | 0 |
| Hev b 3 | 2.53 | 4.40 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hev b 5 | 0 | 0 | 0 | 0 | 0.68 | 0 | 0.57 | 0.53 | 0.50 |
| Hev b 6.02 | 0 | 0 | 0 | 3.43 | 2.80 | 0.50 | 3.13 | 0 | 0 |
The detection limit levels of allergens on FITkit™ were: Hev b 1, 1.2μg/L; Hev b 3, 2.3μg/L; Hev b 5, 0.5μg/L; Hev b 6.02, 0.1μg/L. Values below these detection limits were denoted as zero.
To summarize, in this work it was described a reproducible procedure that allowed the separation of the four most important latex allergens directly from rubber gloves and its enrichment in specific fractions which can potentially be applied for diagnostic or clinical purposes.
Ethical disclosuresProtection of human subjects and animals in researchProtection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this investigation.
Patients’ data protectionConfidentiality of data. The authors declare that no patient data appears in this article.
Right to privacy and informed consentRight to privacy and informed consent. The authors declare that no patient data appears in this article.