The aim of the study is to investigate the levels of toxic heavy metals related with environmental pollution and trace elements involved in antioxidant system in children suffering from recurrent wheezing.
Study DesignOne hundred children with recurrent wheezing (at least three recurrences) between the ages from 1 to 6 years took part in the study, and also 116 age- and sex- matched healthy children were involved in the study as a control group. Venous blood samples were collected and serum mercury, lead, aluminium, zinc, selenium, and copper levels were studied using ICP-MS.
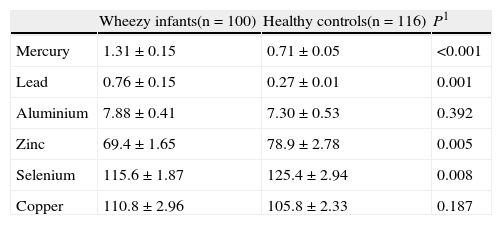
ResultsSerum lead (0.76±0.15 vs. 0.27±0.01, p:0.001) and mercury levels (1.31±0.15 vs 0.71±0.05, p<0.001) were higher in wheezy group than those acquired from the control group. Serum zinc (69.4±1.65 vs. 78.9±2.78, p:0.005) and selenium (115.6±1.87 vs. 125.4±2.94, p:0.008) levels were lower in wheezy group than those acquired from the control group. Serum zinc levels were found to be correlated with number of ARTIs (rp:−0.332, p:0.001) and the number of wheezy attacks (rp:−0.776, p<0.001) during the previous year in the wheezy group.
ConclusionElevated levels of serum lead and mercury and low levels of zinc and selenium may suggest some disturbances in the antioxidant system in children with recurrent wheezing. This means that children with recurrent wheezing are much more susceptible to environmental pollutants and respiratory tract infections than healthy children and this heavy metal-antioxidant relationship may play a role as a contributing factor in the pathogenesis of recurrent wheezing in children.
Acute respiratory tract infections (ARTIs) and chronic respiratory diseases are two of the six global burdens of diseases associated with environmental exposure in children.1 The prevalence of allergic airway diseases has increased dramatically during the past few decades in both industrialised and industrialising countries alike.2 Although genetic factors are important in the development of allergic airways diseases, this rapid increase over recent years can only be explained by the changes that have been observed in the environment.2 Although asthma is a multifactorial disease, an immune/inflammatory response ultimately is followed by the development of allergic inflammatory diseases when the genetically predisposed individual encounters key environmental factors,.3 These environmental factors include infections, allergens, tobacco smoke, and environmental toxicants.4 Mercury and lead are the most common heavy metals readily available in our environment and have been widely used in medicine and in industry.5 Children are exposed to these toxic metals from numerous sources. They may inhale airborne metallic particulates and ingest metals dissolved in food and in drinking water.6 In addition, because of their typically oral behaviour, they are at risk of ingesting heavy metals from painted surfaces and from contaminated dust and soil. As a result of unique physiologic, behavioural, and biological characteristics, children are particularly vulnerable to exposure to environmental toxic metals.4,7 Environmental factors, such as air pollution and exposure to toxic metals, have a contributory effect on the development of recurrent wheezing in young children and the studies carried out in recent years indicate that air pollutants increase the risk of the diseases relating to asthma and wheezing.8
In the context of environmental factors, dietary factors have been proposed to play a role both in the onset and the progression of asthma. Numerous studies suggest that significant decreases in the intake of dietary antioxidants have led to an increased vulnerability of the pulmonary airways to reactive oxygen species and thus this may be an important contributing factor to the increasing incidence of asthma over the last three decades.9 The prevalence of bronchial asthma has been reported to be higher among adults who have had low dietary intake of selenium.10 Furthermore, low dietary intake of selenium during childhood has been thought to be a contributory factor in the development of asthma in later life.11 Several studies reported an association between asthma and low hair and serum/plasma zinc levels, suggesting that asthmatic children were at the risk of zinc deficiency.12,13 Apart from the aforementioned antioxidant characteristics, it has been proposed that a zinc and selenium deficiency switches the Th1 immune response toward a Th2 type response, which, as mentioned earlier, is regarded to be a hallmark of asthma pathophysiology.14–16
In this research, we investigated the levels of toxic heavy metals related to environmental pollution and trace elements involved in oxidative system in children with recurrent wheezing.
Materials and methodsThe subjects were gathered from the paediatric allergy and asthma unit of Keçiören Education and Research Hospital, Ankara, Turkey. This study was conducted between the dates of June 1 and July 31, 2007. One hundred children with recurrent wheezing (at least three recurrences) between the ages of 1 and 6 years were included in the randomised cross-sectional controlled study. None of them had infectious disease or wheezy episode for one month. Children who suffer from malnutrition (weight for age <90% of expected), cystic fibrosis, gastro-oesophageal reflux, pneumonia, tuberculosis or any other chronic illness were excluded. Also 116 age- and sex- matched healthy children were included in the study as a control group. None of them had “ever wheezy” or infectious disease for one month. All the population of this study live in Keçiören. This suburb is one of the developing areas in Ankara. Generally low socio-economic level residents live there. Air pollution is not so high since the heating system is based on natural gas in the centre and there is not much traffic congestion in comparison to other suburbs of Ankara. However, in some parts of Keçiören air pollution in winter has become a serious concern owing to burning cheap coal.
As a part of the study, parents completed a questionnaire about the parental history in order for the physician to diagnose asthma and prenatal maternal smoking status. Parents were asked whether the child's chest had sounded wheezy or had been whistling before, and how frequently the child had wheezed.
After obtaining parental written informed consent, venous blood samples were taken from each subject in each group and serum was separated by centrifugation (Nüve® NF 800) and stored at −80°C until the time of analysis. All samples were studied at the same time to avoid the possible differences that may occur within a day or between days. Serum mercury, lead, aluminium, zinc, selenium and copper levels were studied by using ICP-MS (Agilent, USA).
Statistical analysisDistribution of data was analysed with Leven's t test and expressed as mean±standard error of the mean (SEM). Means and ratios between the two groups were compared using Student's t test and Fisher's exact chi-square test, respectively. Non-parametric statistical methods have been used when the data are distributed unevenly. Pearson Correlation Analysis has been used to determine the relationship of different variants with serum zinc and selenium levels. Statistical significance was regarded significant when two-sided p values were lower than 0.05. All data were processed and analysed on a computer using SPSS 11.0 for Windows.
ResultsOf the 100 children included in the study group, 56 were boys and 44 were girls, with ages ranging from 1 to 5 years (median 27 months). Children in the two groups were similar in terms of their demographic characteristics (Table 1). Serum aluminium and copper levels were not different between the two groups (Table 2). Serum lead and mercury levels were higher in the recurrent wheezy group, according to healthy controls (Table 2). Serum zinc and selenium levels were lower in the recurrent wheezy group, according to healthy controls (Table 2). Serum aluminium and copper levels were not different in the two groups (p>0.05).
Demographic Characteristics of the Study Population.
| Wheezy infants(n=100) | Healthy controls(n=116) | p | |
| Age (months)* | 27.5±1.34 | 30.5±1.13 | 0.0941 |
| Gender | |||
| Male, n (%) | 56 (56) | 68 (58.6) | 0.7832 |
| Female (n, %) | 44 (44) | 48 (41.4) | |
| Height (cm)* | 87.3±1.27 | 90.1±0.87 | 0.1661 |
| Weight (kg)* | 13.5±0.31 | 13.7±0.21 | 0.6671 |
| Skin test positivity (n) | 16 | 0 | <0.0012 |
| Familial history of atopy (n) | 0 | 4 | 0.126 |
| History of tobacco exposure at home (n, %) | 16 (16) | 12 (10.3) | 0.2302 |
| Wheezy attacks during the previous year ¶ | 8 (6-12) | 0 (0-0) | <0.0013 |
| ARTIs during the previous year ¶ | 10 (8-12) | 3 (2-5) | <0.0013 |
| IgE* | 54.9±12.7 | 101.7±23.4 | 0.0991 |
| Eosinophil* | |||
| count/mm3 | 229.5±30.2 | 206.8±11.2 | 0.4521 |
| % | 2.2±0.2 | 2.3±0.1 | 0.7471 |
1t test.
2Chi-square test.
3Mann-Whitney U-test.
*Mean±SEM.
¶ Median (interquartile range).
ARTI: Acute respiratory tract infection.
Serum heavy metals and eser elements levels (mg/dl)*.
| Wheezy infants(n=100) | Healthy controls(n=116) | P1 | |
| Mercury | 1.31±0.15 | 0.71±0.05 | <0.001 |
| Lead | 0.76±0.15 | 0.27±0.01 | 0.001 |
| Aluminium | 7.88±0.41 | 7.30±0.53 | 0.392 |
| Zinc | 69.4±1.65 | 78.9±2.78 | 0.005 |
| Selenium | 115.6±1.87 | 125.4±2.94 | 0.008 |
| Copper | 110.8±2.96 | 105.8±2.33 | 0.187 |
A negative correlation was found between the serum zinc levels and the number of wheezy attacks (rp:−0.776, p<0.001) and the number of acute respiratory tract infections (ARTIs) (rp:−0.332, p:0.001) during the previous year in wheezy group (Figures 1 and 2). Age, weight, height, gender, respiratory infections, and wheezy attacks in the previous year did not show any significant correlation with serum selenium, mercury and lead levels.
The results have demonstrated that children with recurrent wheezing have low levels of zinc and selenium which may protect the lungs by contributing to host antioxidant defence, and they also have higher levels of plasma lead and mercury levels than normal controls, but lead and mercury levels are not at toxic levels. In addition, none of the children included in to the study has symptoms related to lead or mercury toxicity. All the children in the healthy control group and study group in our study live in the same area and this means that the level of exposure to toxic metals in all of the children in this study was to the same degree. The suburb where the study group and the healthy group live is one of the newly developing areas in Ankara. Generally low socio-economic level residents live in this region. Air pollution is not so high because the heating system is based on natural gas in the central part of the district; however, air pollution in winter has become a serious concern owing to the burning of cheap coal. Traffic congestion is also a problem especially in winter in this area. But the study was conducted in summer. Thus, air pollution was not a problem in this season since coal consumption dramatically reduces in summer. These findings demonstrated that high levels of lead and mercury that we have found in our study are not related to high exposure to these metals. According to the findings, we assume that neutralisation of toxic metals may be defective in children with recurrent wheezing. Recent studies indicate that transition metals act as catalysts in the oxidative reactions of biological macromolecules; therefore, the toxicities associated with these metals might be due to oxidative tissue damage.17 Redox-active metals, such as iron, copper and chromium, undergo redox cycling, whereas redox-inactive metals, such as lead, cadmium, mercury and others deplete cells’ major antioxidants, particularly thiol-containing antioxidants and enzymes.17 Either redox-active or redox-inactive metals may cause an increase in the production of reactive oxygen species such as hydroxyl radical, superoxide radical or hydrogen peroxide.17 Enhanced generation of reactive oxygen species can surpass cells’ intrinsic antioxidant defences, resulting in a condition known as “oxidative stress”.17 Therefore, it was speculated that lead and mercury might induce oxidative stress.
In addition to these hazardous effects, it has been demonstrated that lead and mercury also have important effects on the immune system. Heavy metals like lead and mercury are known to inhibit Th1 cells and stimulate Th2 mediated cytokine production and cause an imbalance in the normal Th1:Th2 ratio which plays a major role in the pathogenesis of allergic airway diseases like asthma.3 Furthermore, it has been demonstrated that mercuric chloride enhances IgE-dependent mediator release from human basophiles and suggests that this element may crucially exacerbate IgE-dependent disorders.18 These findings demonstrate that lead and mercury may have a role in asthma and recurrent wheezing pathogenesis. There is no study in the literature examining if lead and mercury have any effects on recurrent wheezing. Furthermore, it has been mentioned that in humans if heavy metals are to be proven to cause an imbalance in the Th1:Th2 ratio; this might explain practitioner reports on the improvements of asthma symptoms after being exposed to heavy metal detoxification.3
It is well known that heavy toxic metals are detoxificated by antioxidant system.17 Besides antioxidant vitamins like A, E and C, some transition elements like zinc or selenium play an important role in this system. Zinc and selenium are the key components of cytoplasmic superoxide dismutases and glutathione peroxidase respectively.19 Given their key roles in the generation and detoxification of reactive oxygen species and the scavenging of free radicals, transition elements like zinc and selenium modulate the response to toxic metals19 and with these antioxidant properties zinc and selenium have significant protective properties over lead and mercury toxicity, respectively.20 It has been demonstrated in a few studies that chronic oxidative stress may be associated with the metal depletion involved in the antioxidant responsive elements.19,21 Regarding what we have mentioned above, we have found in our study that serum levels of zinc and selenium in children with recurrent wheezing are lower than those of the healthy controls. No current study seems to make such comparisons with our study within our knowledge, but in accordance with our findings, it has been demonstrated in one study that hair zinc levels are lower in wheezy children than in healthy controls.22 In another study performed in wheezy children serum selenium levels are found to be lower than in healthy controls and a negative correlation between the serum selenium levels and number of wheezy attacks during the previous year was demonstrated.23 Also zinc and selenium deficiency is known to inhibit Th1 cells and stimulate Th2 type immune response and cause an imbalance in the normal Th1:Th2 ratio like lead and mercury.3,22,23
As well as the significant effects of zinc and selenium on immune response and antioxidant system mentioned above; selenium and zinc stimulate Th1 immune response against viral infections.3,22–23,15 Furthermore, it has been demonstrated that the immune response to viral infections has skewed a Th2-like pattern in selenium and zinc deficiency.3,22,15 Since both asthma and Zn deficiency are associated with a skewing toward the pro-inflammatory Th2 response and an upregulation of the production and release of various pro-inflammatory cytokines, asthmatics who are also zinc deficient are likely to have increased inflammation.14 Prasad also studied how mild zinc deficiency affects the immune system and demonstrated that zinc deficiency caused an imbalance between Th1 and Th2 functions, with decreased production of IL-2, IFN-gamma, and TNF-alpha.24 Prasad has also noted decreased natural killer cell activity and decreased numbers of cytotoxic CD8 T-cell precursor cells.24 Because of these effects of zinc on the immune system, infections of the respiratory tract are more common in zinc deficient individuals.25 In line with this finding, it has been seen in our study that children in recurrent wheezing are inflicted with much more respiratory tract infections. Moreover, serum zinc levels were negatively correlated with ARTIs and wheezy episodes during the previous year. These findings are in line with the literature and have showed that zinc deficiency may play a role in the pathogenesis of frequent wheezy especially due to respiratory viral infections.22–23,15
In conclusion, our results suggest that there may be some disturbances in antioxidant system in children with recurrent wheezing and thus neutralisation of toxic metals like mercury and lead is defective. This means that children with recurrent wheezing are much more susceptible to environmental pollutants than the healthy children are and this heavy metal-antioxidant relationship may play a contributing factor in the pathogenesis of recurrent wheezing in children. Additionally, in countries where low levels of selenium and/or zinc are encountered more frequently, selenium and/or zinc supplementation may help in achieving a better control of wheezing during childhood.
Conflict of interestThe authors have no conflict of interest to declare.