INTRODUCTION
Idiopathic pulmonary haemosiderosis (IPH) is a rare disease characterized by widespread pulmonary bleeding that occurs mainly in infants and children (1). In recent years, many cases previously classified as IPH have been reevaluated, and a diagnosis of microscopic polyangiitis, Goodpasture's syndrome or systemic collagen vascular disorder has been made. However, IPH has been diagnosed in a subset of patients after the microscopic demonstration of haemosiderin-laden macrophages in the alveoli without evidence of capillaritis. The disease has been described in association with milk or gluten sensitivity, although the mechanism linking milk or gluten intolerance to the appearance of alveolar haemorrhage is still elusive (2, 3). Torres et al (4) reported a newborn with cow's milk intolerance and IPH, in which cow's milk feeding could trigger the release of histamine and eosinophil cationic protein (ECP) in peripheral blood. Here, we describe an adult case of IPH, in which a serum histamine-releasing activity (HRA) could be demonstrated during an active phase of the disease.
CASE REPORT
A 30-year-old woman with b -thalassemia minor was admitted to our department because of recurrent episodes of haemoptysis. Since the age of 21 she had been repeatedly hospitalized hor haemoptysis and every time common causes of haemoptysis had been excluded. At the age of 27 she underwent open lung biopsy, and an extensive intra-alveolar haemorrhage was detected, without any inflammatory alteration of lung parenchima. Oral prednisone was started at the dose of 25 mg per day, with remission of pulmonary symptoms. Subsequently, every attempt to reduce prednisone was followed by reappearance of haemoptysis. On admission, infectious causes of recurrent alveolar bleeding were excluded again, as well as systemic vasculitides. Coeliac disease was excluded on the basis of endoscopic and serological findings; specific IgE determination for gliadin, milk proteins and other main food allergens was negative. The disease course was unaffected by a milk-free diet. Then, azathioprine at the dose of 100 mg per day was introduced and, after three months, prednisone was tapered to 10 mg per day, without any further relapse of the disease.
METHODS
During hospitalization, when the patient presented daily haemoptysis, and subsequently, when she was free of recurrent events, blood samples were drawn, and the capacity to induce histamine release was assessed. Leucocyte suspensions were prepared by dextran sedimentation of venous blood taken from two non-atopic donors. After aspiration and centrifugation, leucocytes (with about 7 x 104 basophils, identified as alcian blue positive cells) were resuspended in 100 ml volume of Hepes-buffered saline, and incubated with 100 ml of sera. After incubation for 40 min at 37 °C, the reaction was stopped by addition of 800 ml of ice-cold buffer solution and centrifugation. As control, leucocytes were stimulated also with an optimal dose of polyclonal goat anti-human IgE antiserum (10 mg/ml; Sigma Chemicals, St. Louis, MO, USA) and with sera from 4 normal subjects. The supernatants were assayed for histamine concentration by a commercially available radioimmunoassay (Immunotech, Marseille, France). Total histamine content was obtained by heating the cells at 90 °C for 30 min. Net histamine release was calculate as percentage of total histamine content, after subtraction of spontaneous release.
In order to better characterize the histamine-releasing activity, the effect of heating at 56 °C x1 hour was evaluated. Furthermore, the serum samples from the IPH patient were filtered at 4 °C through an Amicon low-binding, anisotropic, hydrophilic YM membrane with a 100 kDa cut off (Microcon-100 Microconcentrators, Amicon Inc., Beberly, MA, USA). Both fractions < 100 kDa and < 100 kDa were tested for histamine-releasing activity.
RESULTS
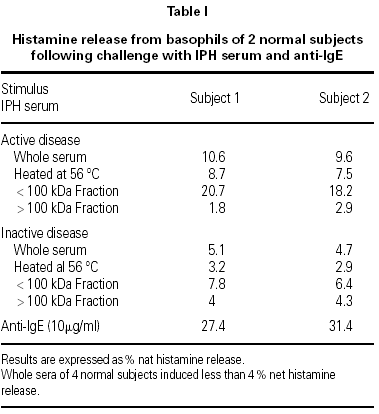
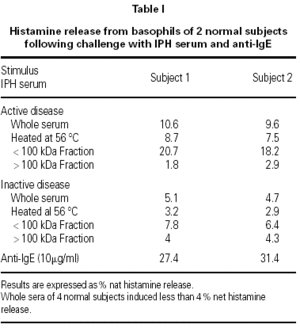
The results, shown in the table I, indicate that the serum sample taken during the active phase of IPH induced 10 % net histamine release from basophils of both donors, whereas the serum sample taken during the remission period induced 5 % histamine release.
Histamine concentration in the serum samples taken during the active phase of the disease and during remission was below 0.5 ng/ml. Sera from 4 normal subjects induced less than 4 % histamine release, whereas anti-IgE induced about 30 % histamine release.
Heating at 56 °C for 1 hour partially reduced the HRA, and filtration through Amicon membrane with a 100 kDa cut off allowed to demonstrate that the HRA resided in the < 100 kDa fractions; the activity was even stronger than that of the whole serum.
DISCUSSION
These findings show that the serum taken from a patient with active IPH can induce histamine release from basophils of healthy donors. Ultrafiltered fractions with a MW above 100 kDa were unable to provoke histamine release, an activity which was retained by the fractions with a MW lower than 100 kDa. Therefore, the serum HRA present in our patient is likely to be a cytokine, and not an immunoglobulin, whose MW is above 100 kDa. In vitro experiments have shown that several cytokines and chemokines with a molecular weight lower than 100 kDa, such as interleukin-3, RANTES, MCP-1 and MCP-3, can induce histamine release from human basophils and act as histamine-releasing factors (5-7).
As yet, chronic idiopathic urticaria is the only human disease in which a circulating histamine-releasing factor has been found and characterized: in most patients the serum HRA is due to an anti FceRI autoantibody or an anti-IgE antibody (8). However, in some cases of chronic idiopathic urticaria a hitherto undefined cytokine colud be involved in the disease (9).
A serum sample taken during the remission phase of IPH had a weaker HRA that the serum sample taken when the disease was active. Therefore, it is concievable that the serum HRA is related to disease activity and immune system activation. The detection of serum HRA in adult patient with IPH is in agreement with the observations reported by Torres et al (4), who found histamine release in blood and urine in a newborn cow's milk intolerance and IPH. In that patient, challenge with cow's milk triggered a late release of histamine and ECP. Since the kinetic of histamine release was not typical of an IgE-mediated reaction, the authors hypothesized that cow's milk feeding could induce the release of cytokines by sensitized T cells, which in turn could activate basophils. The increase of serum HRA detected in our patient during an active phase of the disease suggests that a cytokine-mediated mechanism may underlie the alveolar capillary damage in IPH.